Anti-parallel sheet structures of side-chain-free γ-, δ-, and ε-dipeptides stabilized by benzene–pentafluorobenzene stacking†
Abstract
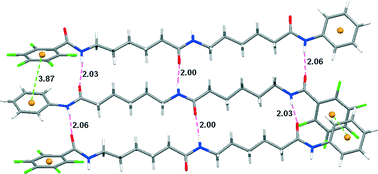
This paper describes an intermolecular benzene–pentafluorobenzene stacking-promoted anti-parallel arrangement of side-chain-free γ-, δ-, and ε-dipeptides. Three diamides have been prepared from γ-, δ-, and ε-amino acids with a benzene ring and a pentafluorobenzene ring attached to their C- and N-terminals, respectively. Their crystal structures showed that all the compounds formed intermolecular benzene–pentafluorobenzene stacking, which guided the molecules to arrange in a ruler-styled pattern and the aliphatic backbones to adopt extended sheet-like conformations. Shorter control compounds also gave rise to a similar intermolecular aromatic stacking, but the central aliphatic amide chains adopted different conformations.

 Please wait while we load your content...
Please wait while we load your content...