Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Click chemistry promoted by graphene supported copper nanomaterials†
Ali
Shaygan Nia
a,
Sravendra
Rana
a,
Diana
Döhler
a,
Xavier
Noirfalise
b,
Alice
Belfiore
b and
Wolfgang H.
Binder
*a
aInstitute of Chemistry, Chair of Macromolecular Chemistry, Faculty of Natural Sciences II (Chemistry, Physics and Mathematics), Martin-Luther University Halle-Wittenberg, von Danckelmann-Platz 4, Halle, 06120, Germany. E-mail: wolfgang.binder@chemie.uni-halle.de; Fax: +49 345 55 27392
bMateria Nova Research Center, 1 Avenue Nicolas Copernic, B7000 Mons, Belgium
First published on 21st October 2014
Abstract
A facile and robust approach is provided for the synthesis of highly dispersed copper nanoparticles immobilized onto graphene nanosheets, useful as a recyclable and reusable heterogeneous catalyst with excellent catalytic activity to achieve Cu(I)-catalyzed [3+2] cycloaddition ‘click’ chemistry.
Since its development by Sharpless1,2 and Meldal,3,4 the Cu(I)-catalyzed [3+2] cycloaddition reaction5 between terminal acetylenes and azides (“click” reaction (CuAAc)) has emerged as a strategy for the rapid and efficient assembly of molecules with diverse functionality on both laboratory and production scales.6–9 Click reactions are modular, tolerant of a wide range of functional groups, simple to perform, insensitive to reaction solvents irrespective to their polar/non-polar or protic/aprotic character.10,11 However, in order to enhance the catalytic activity, the presence of a co-catalyst is required such as bases (mainly amines), auxiliary ligands, and oxidizing or reducing agents depending on the used Cu sources (CuII/Cu0). Furthermore, the recyclability, reusability, and easy removal of the copper catalyst is often severely limited. Therefore, the development of recyclable and stable heterogeneous copper catalysts with improved catalytic activity devoid of any oxidizing/reducing agents is highly desirable.
Herein, we report a facile and robust approach for the synthesis of highly dispersible, recyclable, and reusable Cu(I) nanoparticles decorated onto graphene nanosheets, useful as a catalyst for “click” chemistry without any co-catalyst (Scheme 1). Graphene, a single layer of two-dimensional sp2-hybridized carbon has attracted significant research interest due to its unique electrical and thermal conductivity, including exceptional mechanical, optical and chemical properties.12–14 In contrast to single layer graphene sheets conveniently prepared by mechanical exfoliation, graphene oxide (GO) and reduced graphene oxide (rGO)15,16 are easily available by controlled chemical reactions and can be produced on a large scale. Also due to the presence of functional groups on GO and rGO surfaces, further chemical reaction onto their surfaces is possible. On account of their large surface area, their unique interaction with metal particles,17,18 and their performance for electron capture, transport, as well as prevention of supported nanoparticles agglomeration due to their scaffold behaviour, graphene supported catalysts have represented outstanding catalytic activity compared to other carbon supported catalysts.19 Several reports for the preparation of different metal particles-GO heterostructures and their wide range of applications from photocatalytic hydrogen production to lithium ion batteries have been discussed.20,21 Recently, few reports have appeared regarding the preparation of Cu–GO nanoconjugates and their relevance in hydrogen generation, supercapacitors, as well as in other applications.22–25 However according to our knowledge their application in CuAAc catalyzed “click” chemistry has not been explored yet.
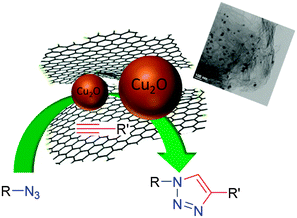 | ||
| Scheme 1 Schematic illustration of click chemistry promoted by graphene supported copper nanoparticles. | ||
For the preparation of graphene oxide (GO), Hummer's method was applied,26 whereas ion exchange with Cu2+ was achieved by dispersing GO in water,27 followed by addition of copper(II) acetate under vigorous stirring for overnight. Afterwards Cu(II)–GO was reduced under Ar at 600 °C in an oven to obtain TRGO/Cu(I). We first investigated the morphology and chemical composition of our nanoconjugates by TEM, EDX, XPS, and FAAS.
Fig. 1a shows the TEM image of TRGO–Cu(I) conjugates, where highly dispersed uniformly sized Cu nanoparticles on the surface of graphene nanosheets were obtained. From the TEM images the average particle size is about 25 nm for Cu particles (Fig. 1b). HRTEM was performed to measure the lattice plane distance in a single Cu nanoparticle (Fig. 1c and d), where 0.2385 ± 0.01 nm as a lattice plane distance was obtained, that could be due to the presence of CuO(110)28 or Cu2O(111),29 indicative that for the TRGO–Cu conjugates the Cu is in the form of Cu(I) or Cu(II). To confirm the existing form of Cu, quantitation of nanoparticles was also achieved by STEM-EDXS (Fig. S1, ESI†) indicating that Cu and O are present in ratio of 68.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31.2 (≈2
31.2 (≈2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
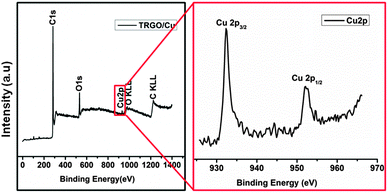
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which strongly supports that the particles consist of Cu2O, and the valency of Cu is Cu(I) as required for their use in “click” chemistry. Further evidence of the chemical composition of the nanoconjugates was obtained by X-ray photoelectron spectroscopy (XPS) (Fig. 2a), where the peaks correspond to C 1s (285.1 eV), O 1s (531.6 eV) and Cu 2p. The high-resolution XPS (Fig. 2b) reveals that Cu 2p3/2 peaks located at 932.37 eV and 952.31 eV correspond to the peak energy of Cu(I).30 Also there are no peak satellites at 942 eV and 962 eV and no peaks at 934.8 eV which would be related to Cu(II). The Cu content of the sample was also determined by analysis on a flame atomic absorption spectroscopy (FAAS) as 7.55 × 10−7 mol mg−1 loading.
1), which strongly supports that the particles consist of Cu2O, and the valency of Cu is Cu(I) as required for their use in “click” chemistry. Further evidence of the chemical composition of the nanoconjugates was obtained by X-ray photoelectron spectroscopy (XPS) (Fig. 2a), where the peaks correspond to C 1s (285.1 eV), O 1s (531.6 eV) and Cu 2p. The high-resolution XPS (Fig. 2b) reveals that Cu 2p3/2 peaks located at 932.37 eV and 952.31 eV correspond to the peak energy of Cu(I).30 Also there are no peak satellites at 942 eV and 962 eV and no peaks at 934.8 eV which would be related to Cu(II). The Cu content of the sample was also determined by analysis on a flame atomic absorption spectroscopy (FAAS) as 7.55 × 10−7 mol mg−1 loading.
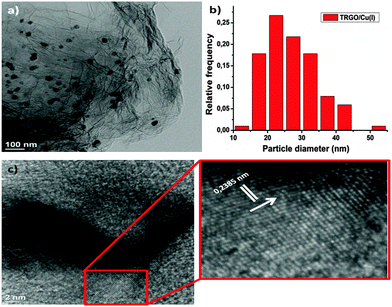 | ||
| Fig. 1 (a) TEM image of the TRGO/Cu catalyst. (b) Copper particles size and distribution. (c, d) HRTEM of TRGO/Cu(I) and calculation of the lattice plane distance. | ||
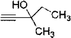
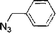
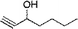
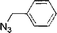
The catalytic activity of the TRGO/Cu catalyst was investigated in the azide/alkyne click chemistry between benzyl azide and phenyl acetylene under different reaction conditions in high yields (up to 99%) as a model system. Also the model reaction for commercial available Cu on charcoal (Cu/C)31 and Cu2O powder as a reference was done, but a very low click conversion was obtained by these commercial catalysts (Table 1).
| Entry | Conditions | Conversion |
|---|---|---|
| 1 | TRGO without copper, in THF, 40 °C, 48 h | 0 |
| 2 | Copper on charcoal (2 mol%), in THF, 40 °C, 48 h | 3 |
| 3 | Cu2O (2 mol%), in THF, 40 °C, 48 h | 0 |
| 4 | TRGO/Cu (2 mol%), in THF, 40 °C, 48 h | 99 |
| 5 | TRGO/Cu (2 mol%), in THF, room temperature, 48 h | 70 |
| 6 | Recycled catalyst in air (first cycle) | 99 |
| 7 | Recycled catalyst in air (fourth cycle) | 55 |
| 8 | TRGO/Cu, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/methanol, room temperature, 24 h 1 water/methanol, room temperature, 24 h |
99 |
The recyclability of the catalyst was studied using the same alkyne/azide click model reactants. After four repetitions, a reduction (∼30%) of the reaction yield was observed (Fig. 3a), whereas the washing and recycling of the catalyst was accomplished in open air environment, supportive of the stability and recyclability of the synthesized catalyst (Table 1, Fig. 3a). The reduction of reaction yield after several repetitions could be due to agglomeration of Cu particles, as observed by TEM (Fig. 3b).
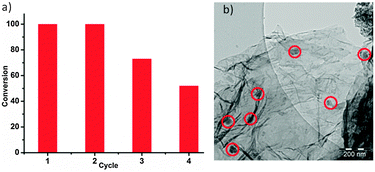 | ||
| Fig. 3 (a) Catalyst recycling and stability in four cycles of click chemistry. (b) TEM image of Cu particles after the fourth run. | ||
For kinetic investigation, NMR studies have been performed. Therefore aliquots were taken at intervals from the reaction, filtered to remove the catalyst and conversion of the reaction was determined by the resonance of the CH2-moiety of benzyl azide (educt) in comparison to the CH2-moiety of the click product (Fig. S2, ESI†). Eqn (1)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 was used for the kinetic study and calculation yielded k′ = 1.33 M−1.3 h−1, which was in the same range as those reported previously by Finn et al.33 using CuSO4 and sodium ascorbate as catalytic system.
32 was used for the kinetic study and calculation yielded k′ = 1.33 M−1.3 h−1, which was in the same range as those reported previously by Finn et al.33 using CuSO4 and sodium ascorbate as catalytic system.
| rate = k′ [alkyne]1.3±0.2[azide]1±0.2 (k′ = k[Cu]2) | (1) |
To evaluate the performance of synthesized TRGO/Cu catalyst for bulk click chemistry including high molecular weight molecules, melt rheology was carried out for trivalent azide- and alkyne-functionalized polyisobutylenes (PIBs, Mn 5500 g mol−1 and 6900 g mol−1 respectively) (Scheme 2), suitable for crosslinking via “click” chemistry at room temperature, being designed for self healing materials.9 For crosslinking experiments an azide-functionalized trivalent PIB and an alkyne-functionalized trivalent PIB were mixed with the TRGO/Cu catalyst (2 mol%, 0.7 mass%) and crosslinking was investigated on a rheometer plate. Fig. S3a† shows the rheology result of the “click” reaction of polymers at 20 °C, where at the beginning of the reaction, the sample clearly possesses liquid like character; the loss modulus is higher than the storage modulus (G′′ > G′). After some time (520 minutes), both moduli increase and a crossover was observed (gel point), which is indicative of network formation. The structure of polymer-TRGO–Cu mixtures before and after crosslinking was investigated using FT-IR measurements (Fig. S3b, ESI†), where after crosslinking a complete disappearance of the azide peak (2097 cm−1) confirms the complete crosslinking of the polymer. In contrast, in experiments with Cu/C and without catalyst no gel point was observed even after 1000 minutes, which again supports the high activity of the synthesized TRGO/Cu catalyst.
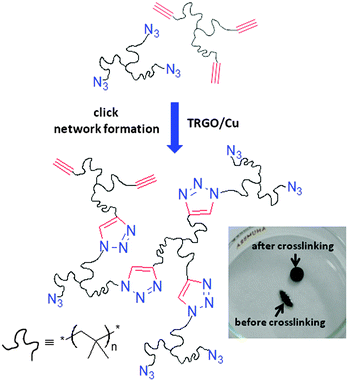 | ||
| Scheme 2 Schematic illustration of the bulk click reaction of PIB-azide and PIB-alkyne with TRGO/Cu(I) at 20 °C via melt rheology. | ||
In summary, we have presented a facile and robust approach for the immobilization of Cu(I) catalyst onto graphene nanosheets obtaining a highly active TRGO/Cu(I)-catalyst for the Cu(I)-catalyzed alkyne-azide “click” cycloaddition reaction. The TRGO/Cu-catalyst shows high stability at standard reaction conditions (air) with excellent recyclability and reusability. Furthermore, the TRGO/Cu catalyst shows excellent performance for bulk click reactions as proven via melt-rheology. Thus, Cu nanoparticles immobilized onto graphene nanosheets can function as an effective catalyst in click chemistry under both solvent and bulk conditions, including low and high molecular weight molecules.
We gratefully acknowledge the financial support from the EU-IASS project: Improving the Aircraft Safety by Self-Healing Structure and Protecting Nanofillers, European Union Seventh Framework Programme (FP7/20072013), Grant Agreement No. 313978 as well as the grant DFG-BI 1337/8-1 and DFG-BI 1337/8-2 within the SPP 1568 (“Design and Generic Principles of Self-Healing Materials).
Notes and references
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- M. T. Meldal and C. W. Tornøe, Proceedings of the Second International and the Seventeenth American Peptide Symposium, 2001, pp. 263–264.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef PubMed.
- R. Huisgen, Proc. Chem. Soc., 1961, 357–396 Search PubMed.
- W. H. Binder and R. Zirbs, Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc., 2002 Search PubMed.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS.
- B. Uszczynska, T. Ratajczak, E. Frydrych, H. Maciejewski, M. Figlerowicz, W. T. Markiewicz and M. K. Chmielewski, Lab Chip, 2012, 12, 1151–1156 RSC.
- D. Döhler, P. Michael and W. H. Binder, Macromolecules, 2012, 45, 3335–3345 CrossRef.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS PubMed.
- J.-F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018–1025 CrossRef CAS PubMed.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. Yang, R. E. Bachman, X. Feng and K. Müllen, Acc. Chem. Res., 2012, 46, 116–128 CrossRef PubMed.
- S. Eigler, S. Grimm, F. Hof and A. Hirsch, J. Mater. Chem. A, 2013, 1, 11559–11562 CAS.
- S. Eigler and A. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 7720–7738 CrossRef CAS PubMed.
- E. Yoo, T. Okata, T. Akita, M. Kohyama, J. Nakamura and I. Honma, Nano Lett., 2009, 9, 2255–2259 CrossRef CAS PubMed.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mülhaupt, J. Am. Chem. Soc., 2009, 131, 8262–8270 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- J. Qin, W. Lv, Z. Li, B. Li, F. Kang and Q.-H. Yang, Chem. Commun., 2014, 50, 13447–13450 RSC.
- T. Jia, A. Kolpin, C. Ma, R. C.-T. Chan, W.-M. Kwok and S. C. E. Tsang, Chem. Commun., 2014, 50, 1185–1188 RSC.
- P. D. Tran, S. K. Batabyal, S. S. Pramana, J. Barber, L. H. Wong and S. C. J. Loo, Nanoscale, 2012, 4, 3875–3878 RSC.
- S. K. Movahed, M. Dabiri and A. Bazgir, Appl. Catal., A, 2014, 481, 79–88 CrossRef CAS PubMed.
- C. Hong, X. Jin, J. Totleben, J. Lohrman, E. Harak, B. Subramaniam, R. V. Chaudhari and S. Ren, J. Mater. Chem. A, 2014, 2, 7147–7151 CAS.
- X. Dong, K. Wang, C. Zhao, X. Qian, S. Chen, Z. Li, H. Liu and S. Dou, J. Alloys Compd., 2014, 586, 745–753 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- T. Kyotani, K.-y. Suzuki, H. Yamashita and A. Tomita, Tanso, 1993, 1993, 255–265 CrossRef.
- O. García-Martínez, R. M. Rojas, E. Vila and J. L. M. de Vidales, Solid State Ionics, 1993, 63–65, 442–449 CrossRef.
- M. L. Foo, Q. Huang, J. W. Lynn, W.-L. Lee, T. Klimczuk, I. S. Hagemann, N. P. Ong and R. J. Cava, J. Solid State Chem., 2006, 179, 563–572 CrossRef CAS PubMed.
- S. Suzuki, M. Saito, S. C. Kang, K. T. Jacob and Y. Waseda, Mater. Trans., JIM, 1998, 39, 1024–1028 CrossRef CAS.
- B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235–8238 CrossRef CAS PubMed.
- V. O. Rodionov, V. V. Fokin and M. G. Finn, Angew. Chem., 2005, 117, 2250–2255 CrossRef.
- V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Finn, J. Am. Chem. Soc., 2007, 129, 12696–12704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supporting data. See DOI: 10.1039/c4cc07774a |
| This journal is © The Royal Society of Chemistry 2014 |