The effect of π-conjugation in the macrocyclic ring on the photophysical properties of a series of thiaaceneporphyrinoids†
Abstract
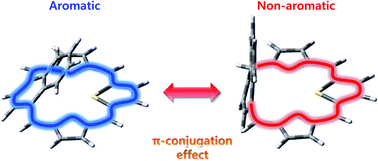
In a series of thiaaceneporphyrinoids, their conformers exhibit macrocyclic π-conjugation pathways controlled by a dihedral angle between the porphyrin framework and acene planes. Conformational equilibria significantly affect the photophysical properties of these macrocycles.

 Please wait while we load your content...
Please wait while we load your content...