Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Transforming polyethylenimine into a pH-switchable hydrogel by additional supramolecular interactions†
M.
Fleischer
and
C.
Schmuck
*
Institute of Organic Chemistry, University of Duisburg-Essen, Universitaetsstrasse 7, 45141 Essen, Germany. E-mail: carsten.schmuck@uni-due.de
First published on 23rd July 2014
Abstract
Attaching a self-complementary zwitterionic supramolecular binding site to the amino groups of polyethyleneimine leads to a pH switchable hydrogel as dimerization of the zwitterions introduces additional crosslinks between the polymer chains. This gel responds to both the addition of either acid or base, as only the zwitterion can self-assemble but neither the protonated or deprotonated form.
Supramolecular gels1,2 and polymers3 have become a topic of increasing interest in recent years. Compared to traditional polymers, in which the individual monomers are linked irreversibly and thus permanently by covalent bonds during polymer synthesis, supramolecular linkages are reversible, which can result in new dynamic structures. The individual building blocks are thus held together by rather weak noncovalent interactions such as hydrogen bonds, electrostatic interactions or aromatic stacking, respectively. This can provide supramolecular polymers with interesting and new properties which are rather difficult to achieve in traditional polymers.4 For example, the polymer can be self-healing as the system in principle is always close to the thermodynamic equilibrium. Structural defects can therefore be healed in time by reorganization of the noncovalent linkages between the monomers. The material can also become switchable, when the supramolecular interaction, which holds the monomers together, responds to changes in the surrounding (e.g. change in solvent composition, pH, addition/removal of metalions etc.).5 For the development of “smart materials” this is a valuable benefit which is not or much more difficultly achieved with classical polymers. However, the challenge for supramolecular polymers is to realize a large enough degree of polymerization to actually observe macroscopic polymeric properties. The polymerization degree directly depends on the concentration of the monomer and on the strength of the noncovalent interaction which holds the monomers together.6–8 As these interactions are significantly weaker than covalent bonds (typically less than 50 kJ mol−1) often only oligomers rather than polymers are present in solution. For example, hydrogen bonds are among the most often used noncovalent interaction in supramolecular polymers. They however only function in nonpolar organic solvents whereas in polar and especially aqueous solvents they become so weak that no polymerization occurs.9
To circumvent this problem one can combine both types of polymers by introducing additional supramolecular binding sites into classical covalent polymers. The covalent polymer thus provides certain macroscopic polymeric properties which are then further modulated by the supramolecular interactions. As the later can be switched by external stimuli, the whole material becomes switchable as well. This approach avoids the necessity to build a stable, long polymer just by weak noncovalent interactions alone, but it retains the advantages which these interactions introduce into the material.
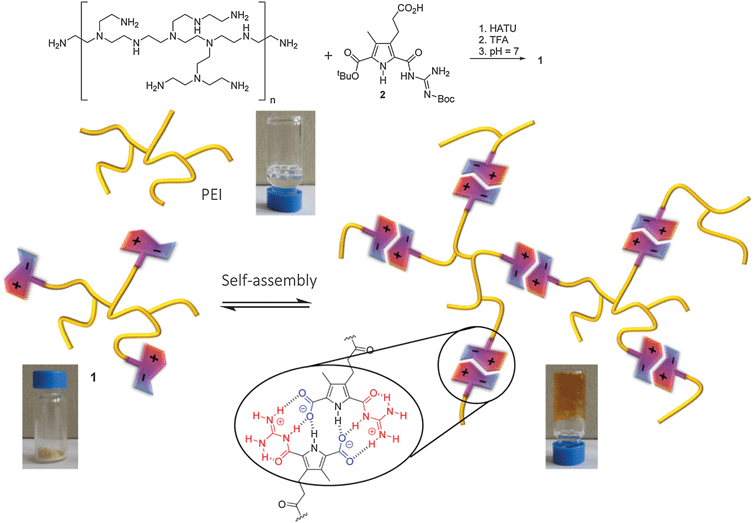
In this report we demonstrate that polyethylene imine (PEI), a commercially available polymer, can be transformed into a pH-switchable hydrogel by addition of a self-complementary zwitterionic binding site into the polymer. PEI can be synthesized industrially by ring-opening polymerization of aziridine. Depending on the conditions used it is a branched polymer which contains varying numbers of primary, secondary and tertiary amino groups. Branched PEI is a viscous liquid which is well soluble in water. Due to its large numbers of positive charges at neutral pH PEI is used for example in microbiology as an artificial gene transfection vector.10 However, it is rather cytotoxic preventing its application in medicine. We reasoned that by functionalizing e.g. the primary amino groups with a self-complementary guanidiniocarbonyl pyrrole carboxylate zwitterion (GCP-zwitterion) we should be able to introduce pH-switchable reversible crosslinks between the polymer chains which should significantly alter the polymer properties. The GCP zwitterion was developed by us a few years ago and forms extremely stable head-to-tail dimers held together by H-bond enforced ionpairs. For example in DMSO this dimer has a stability of Kdimca. 1010 M−1, and even in pure water dimerization occurs (Kdimca. 102 M−1).11 We have already used this zwitterion for the formation of different types of supramolecular oligomers and polymers.12
As the zwitterion is only present in a pH range of ca. 5–8, both protonation as well as deprotonation destroys its self-complementarity providing such materials with a dual pH-responsiveness not achievable by simple ionpair formation alone. Introducing this zwitterionic binding site as additional sticky ends into PEI should enhance the degree of three-dimensional crosslinks within the polymer making the polymer more stable and robust (Scheme 1) but only around neutral pH.
The synthesis of this new hybrid material 1 was performed by amide bond formation between PEI and an excess of a protected precursor of the zwitterion which contains an additional carboxylic acid functionality in the periphery using HATU as the coupling reagent (details see ESI†). Deprotection of the zwitterionic binding site was achieved by treatment of the functionalized polymers with trifluoroacetic acid. After pH adjustment to pH = 7 and dialysis to remove excess salts, the desired zwitterion functionalized PEI 1 was obtained as a beige solid (Scheme 1) in contrast to the unfunctionalized PEI which is a liquid. Obviously the introduction of the GCP zwitterions significantly altered the properties of the polymer as hoped for. The loading of the polymer with zwitterionic binding sites was determined using the characteristic UV absorption of the GCP group. Based on the assumption that the molar absorbance ε of the protected GCP group in the precursor before coupling is the same as in the functionalized polymer (which is reasonable as the UV absorbance is due to the pyrrole moiety which is not affected electronically by coupling the precursor to the polymer) a loading of ca. 40% was determined (details see ESI†). This loading was also confirmed by GPC measurements. The functionalized polymer has a molecular weight of Mw ≈ 46.100 g mol−1, which is significantly larger than for the starting PEI (Mw ≈ 22.700 g mol−1). This increase in molecular weight is due to the attached GCP moieties which have a molecular mass of 265 g mol−1. This means that on average 88 GCP moieties are present within each polymer. As each polymer has on average 220 terminal amino groups this results in a loading of 40%, which is in perfect agreement with the results from quantitative analysis of the UV absorbance.
The influence of these zwitterions on the polymer was already obvious from the altered appearance of the material, which is a solid and not a liquid anymore. The solid was well soluble in water. The resulting solutions are more viscous than corresponding solutions of the unfunctionalized PEI of the same concentrations (Fig. 1). Whereas the viscosity of aqueous solutions of PEI increases more or less linearly with increasing concentrations, the viscosity of corresponding solutions of 1 not only have a significantly steeper increase but above ca. 3 mM the viscosity increases exponentially until at a concentration >160 mg mL−1 the solution turned into a stable hydrogel. This indicated that indeed the zwitterionic binding sites form additional sticky ends which upon dimerization introduce further supramolecular crosslinks into the polymer. The viscoelastic properties of the hydrogel were studied by dynamic rheology. A frequency sweep (at 0.1% strain, [1] = 170 mg mL−1) exhibited higher values of G′ than G′′ in the whole frequency range (ESI†). This is a typical behaviour for a gel. The stiffness/rigidity value, the ratio of the two dynamic moduli G′/G′′, is ca. 5–6 indicating a rather stable supramolecular gel.13
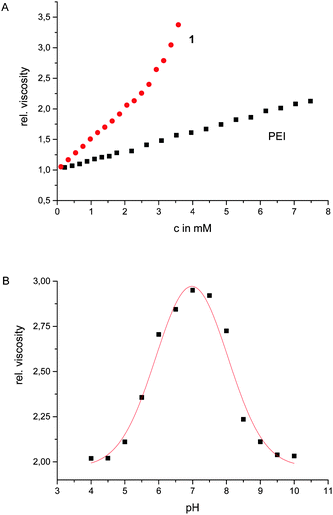 | ||
| Fig. 1 Relative viscosity of aqueous solutions of 1 and PEI with varying concentrations (A); relative viscosity of 1 at different pH values ([1] = 150 mg mL−1) (B). | ||
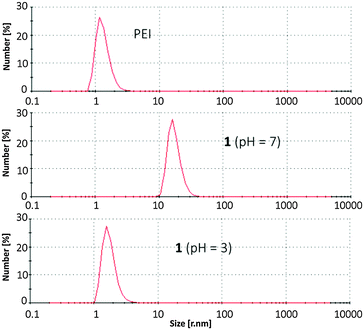
Microscopic images were used to further characterize the gel. Solutions of functionalized and nonfunctionalized PEI were spin coated onto freshly cleaved mica and analysed by atomic force microscopy (AFM) in noncontact tapping mode. Whereas PEI itself forms spherical aggregates of varying size on the surface (Fig. 2A), the hybrid material appears as long compact polymer strands (Fig. 2B). Also field emission scanning electron microscopy (FESEM, Fig. 2D) of dried samples of the hydrogel showed linear fibres which form an entangled network as expected for a stable gel. Dynamic light scattering (DLS) of aqueous solutions also confirmed the formation of large supramolecular aggregates (Fig. 3). Solutions of PEI itself show the presence of particles with a hydrodynamic radius of ca. 1.3 nm (calculated assuming spherical particles). In contrast to this the hydrogel at pH 7 forms significantly larger aggregates of ca. 17.4 nm radius. The hydrodynamic radius has thus increased by a factor of 10 by functionalization with the zwitterions. These large aggregates are obviously formed by supramolecular interactions between zwitterions in different polymer chains, as the additional crosslinks increase the polymerization degree and therefore the apparent size of the material as observed in the DLS measurements.
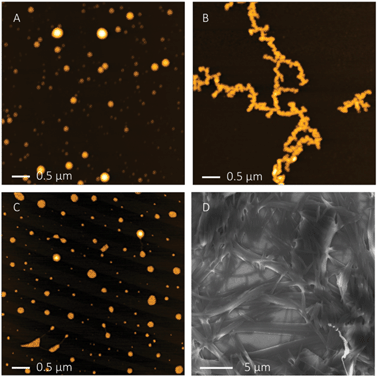 | ||
| Fig. 2 AFM height images of PEI (A), 1 at pH = 7 (B) and pH = 3 (C) ([1] = 2 mg mL−1) and FESEM image of 1 (D) ([1] = 1.7 mg mL−1). | ||
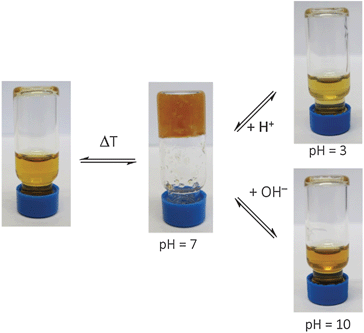
However, as explained in the beginning, the self-complementary zwitterion is only present in the pH range of ca. 5–8 due to the pKa-values of the carboxylic acid (pKa 5) and the guanidiniocarbonyl pyrrole cation (pKa 7). Below or above these pH values the zwitterion is either protonated or deprotonated. The resulting cation or anion, respectively, is not self-complementary anymore. Hence, the supramolecular crosslinking is turned-off. Accordingly at pH = 3 DLS provides a hydrodynamic radius of 1.7 nm for the functionalized polymer 1 which is only slightly larger than the size of the nonfunctionalized PEI reflecting the increased molar mass of 1 compared to PEI. AFM images of the functionalized polymer 1 at pH 3 no longer show any strands and networks but the same type of spherical aggregates as seen for PEI (Fig. 2C). Both experiments confirm that the supramolecular crosslinks can be switched off by protonation of the zwitterion. The same is observed upon deprotonation at pH = 10 (ESI†). This pH responsiveness is also visible by naked eye. The hydrogel is turned into a liquid both by the addition of acid or base (Fig. 4). As for any supramolecular polymer the gel also becomes liquid at elevated temperature. Importantly, these changes are fully reversible and the hydrogel is restored upon cooling or readjustment to the pH of 7.
We have shown here that additional supramolecular crosslinks within a conventional polymer (PEI) can significantly alter the macroscopic properties of the material; in this case turning a viscous liquid into a stable hydrogel. Furthermore, the supramolecular interaction which is due to the dimerization of a self-complementary zwitterion can be switched on and off by external stimuli (temperature, pH) which provides the gel with a dual pH-responsiveness. Such hybrid-materials which combine the features of covalent and supramolecular polymers might be interesting for the development of smart functional materials. For example, the reversible supramolecular linkages introduce responsiveness to external stimuli which purely covalently linked materials do not possess and which can be used to switch the material between to different states (e.g. functional/non-functional).14
We thank Prof. Dr T. Govindaraju (Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), Bangalore, India) for the FESEM measurement, Dr Christoph Hirschhäuser (University of Duisburg-Essen) for helpful comments and the DFG (Deutsche Forschungsgemeinschaft) for funding.
Notes and references
- S. Dong, B. Zheng, D. Xu, X. Yan, M. Zhang and F. Huang, Adv. Mater., 2012, 24, 3191 CrossRef CAS PubMed.
- X. Yan, D. Xu, X. Chi, J. Chen, S. Dong, X. Ding, Y. Yu and F. Huang, Adv. Mater., 2012, 24, 362 CrossRef CAS PubMed.
- (a) T. F. A. De Greef and E. W. Meijer, Nature, 2008, 453, 171 CrossRef CAS PubMed; (b) D. C. Sherrington and K. A. Taskinen, Chem. Soc. Rev., 2001, 30, 83 RSC; (c) B. J. B. Brunsveld, E. W. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef PubMed.
- (a) A. Noro, M. Hayashi and Y. Matsushita, Soft Matter, 2012, 8, 6416 RSC; (b) E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195 RSC.
- (a) M. D. Segarra-Maset, V. J. Nebot, J. F. Miravet and B. Escuder, Chem. Soc. Rev., 2013, 42, 7086 RSC; (b) T. Fenske, H.-G. Korth, A. Mohr and C. Schmuck, Chem. – Eur. J., 2012, 18, 738 CrossRef CAS PubMed; (c) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC.
- (a) A. Ciferri, Macromol. Rapid Commun., 2002, 23, 511 CrossRef CAS; (b) T. F. A. de Greef, M. M. J. Smudlers, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef CAS PubMed; (c) A. T. ten Cate and R. P. Sijbesma, Macromol. Rapid Commun., 2002, 23, 1094 CrossRef CAS.
- S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed.
- M. Zhang, D. Xu, X. Yan, J. Chen, S. Dong, B. Zheng and F. Huang, Angew. Chem., Int. Ed., 2012, 51, 7011 CrossRef CAS PubMed.
- (a) G. A. Jeffrey, An introduction to hydrogen bonding, Oxford university press, 1997 Search PubMed; (b) C. A. Hunter, Angew. Chem., Int. Ed., 2004, 43, 5310 CrossRef CAS PubMed; (c) G. V. Oshovsky, D. N. Reinhoudt and W. Verboom, Angew. Chem., Int. Ed., 2007, 46, 2366 CrossRef CAS PubMed.
- (a) O. Boussif, F. Lezoualch, M. A. Zanta, M. D. Mergny, D. Scherman, B. Demeneix and J. P. Behr, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 7297 CrossRef CAS; (b) D. W. Pack, A. S. Hoffman, S. Pun and P. S. Stayton, Nat. Rev. Drug Discovery, 2005, 4, 581 CrossRef CAS PubMed; (c) M. A. Minzer and E. E. Simanek, Chem. Rev., 2009, 109, 259 CrossRef PubMed.
- C. Schmuck and W. Wienand, J. Am. Chem. Soc., 2003, 125, 452 CrossRef CAS PubMed.
- (a) Y. Hisamatsu, S. Banerjee, M. B. Avinash, T. Govindaraju and C. Schmuck, Angew. Chem., Int. Ed., 2013, 52, 12550 CrossRef CAS PubMed; (b) M. T. Fenske, W. Meyer-Zaika, H.-G. Korth, H. Vieker, A. Turchanin and C. Schmuck, J. Am. Chem. Soc., 2013, 135, 8342 CrossRef CAS PubMed; (c) T. H. Rehm, F. Gröhn and C. Schmuck, Soft Matter, 2012, 8, 3154 RSC; (d) G. Gröger, W. Meyer-Zaika, C. Böttcher, F. Gröhn, C. Ruthard and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 8961 CrossRef PubMed.
- (a) S. Seiffert and J. Sprakel, Chem. Soc. Rev., 2012, 41, 909 RSC; (b) G. Yu, X. Yan, C. Han and F. Huang, Chem. Soc. Rev., 2013, 42, 6697 RSC.
- E. Krieg, H. Weissman, E. Shirman, E. Shimoni and B. Rybtchinski, Nat. Nanotechnol., 2011, 6, 141 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, spectroscopic characterization, details of the performed characterization studies. See DOI: 10.1039/c4cc03281k |
| This journal is © The Royal Society of Chemistry 2014 |