Open Access Article
Open Access ArticleZn(II) and Cu(II) complexes of a new thiophene-based salphen-type ligand: solution-processable high-performance field-effect transistor materials†
Ashish K.
Asatkar
a,
Satyaprasad P.
Senanayak
b,
Anjan
Bedi
a,
Snigdha
Panda
a,
K. S.
Narayan
*b and
Sanjio S.
Zade
*a
aDepartment of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Kolkata, Mohanpur Campus, Mohanpur-741252, India. E-mail: sanjiozade@iiserkol.ac.in
bChemistry and Physics of Materials Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Bangalore 560064, Karnataka, India. E-mail: narayan@jncasr.ac.in
First published on 8th April 2014
Abstract
A new thiophene-based salphen-type ligand, 1, and its Cu(II) and Zn(II) complexes, 1·Cu and 1·Zn·MeOH, were designed and synthesized. These metal organic complexes (MOCs), with ultra-close π-stacking interactions were explored as a novel class of active materials for organic field effect transistors (OFETs). The top-contact bottom gated OFETs fabricated from solution-processed thin films of 1·Cu and 1·Zn·MeOH exhibited excellent p-type mobilities (up to 0.7 and 1.5 cm2 V−1 s−1, respectively).
Development of semiconducting materials for OFETs has become an area of current research.1 Major challenges in this field include the development of cheaper, solution-processable and high conducting materials, with low temperature device fabrications for light-weight, flexible devices. The proper ordering of molecules possessing π–π interactions is quite important for facile charge transport in OFET materials.2
Salen/salphen are Schiff-base ligands prepared from two equivalents of salicylaldehyde with one equivalent of 1,2-diaminobenzene/ethylenediamine, affording a potential N2O2 tetradentate chelating system to form square planar, square pyramidal and octahedral complexes. In square planar and square pyramidal complexes, the ligand frameworks adopt nearly planar geometries due to coordination with a metal, and they thus afford strong intermolecular π–π interactions. Driven by the ease of the synthesis of these salen/salphen based metal complexes, and their versatile potential applications in catalysis, biological studies, sensory materials, molecular magnetism and materials science,3 there has been a tremendous growth in the research on this class of materials in last few decades.4 Thiophene-capped salen/salphen complexes were explored to obtain metalloorganic conducting polymers for their applications in solution-processable electronics.5
It is fundamentally important to understand the role of metal-atoms in the framework of electrically functional conjugated systems. Important examples of such materials include metal–dithiolene complexes and metal–phthalocyanines.6 Vacuum deposited thin films of phthalocyanine based vanadium and titanium complexes have shown appreciable results.7 However, the devices of these complexes were constructed using a vacuum deposition technique, which is not compatible with low cost, flexible electronic applications.
In this communication, we demonstrate FETs based on solution processable Cu(II) and Zn(II) complexes of thiophene-based salphen-type ligands with p-type mobilities as high as ∼0.7 and 1.5 cm2 V−1 s−1 respectively. Additionally, these field effect mobility (μFET) values were obtained from active films, which did not require any high temperature annealing processes. Such high performing solution-processable metal organic complexes (MOCs) open up a new family of materials for applications in large surface area based electroactive applications, which include flexible electronics, bio-electronics and optoelectronics. The ease of synthesis of these MOCs, and the use of abundant and cheaper metals like copper and zinc, will be ideal for the low-cost production of large surface area flexible electronic devices.
The basic idea behind replacing salicylaldehyde with 2-formyl-3-hydroxythiophene to construct these new salphen-type ligands, was to employ ultra-close intermolecular π–π interactions in addition to S⋯S interactions in the solid state for the resulting complexes. This can facilitate effective charge transport in FET devices. Additionally, the incorporation of thiophene rings directly into the coordination-sphere of the MOCs affords potential precursors for metalloorganic conjugated polymers.8
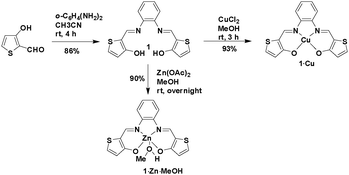
New Schiff base ligand 1 was synthesized in high yield by the coupling of 2-formyl-3-hydroxythiophene and 1,2-diaminobenzene in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in dry acetonitrile at room temperature (Scheme 1). Reactions of ligand 1 with CuCl2 and Zn(OAc)2 in a 1
1 molar ratio in dry acetonitrile at room temperature (Scheme 1). Reactions of ligand 1 with CuCl2 and Zn(OAc)2 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in dry methanol at room temperature afforded the neutral complexes 1·Cu and 1·Zn·MeOH, respectively, with excellent yields.
1 molar ratio in dry methanol at room temperature afforded the neutral complexes 1·Cu and 1·Zn·MeOH, respectively, with excellent yields.
Elemental and ESI-MS analyses of the Cu(II) and Zn(II) complexes indicated the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
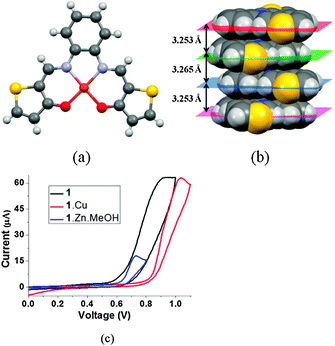
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand–metal complexes 1·Cu and 1·Zn·MeOH. 1H and 13C NMR spectra of complex 1·Zn·MeOH in DMSO-d6 revealed the presence of metal-coordinated MeOH molecules, suggesting a square pyramidal geometry around the metal center. It also indicated that the MeOH remained coordinated to the Zn even in the presence of DMSO. All attempts to grow single crystals of complex 1·Zn·MeOH, suitable for single crystal X-ray diffraction (SCXRD) analysis, were unsuccessful. Complex 1·Cu crystallizes with the acentric monoclinic space group C2/c. The complex has a square planar geometry around the metal center with a dihedral angle between the Cu–N(1)–O(2) and the Cu–N(2)–O(1) planes of 10.144(78)° (Fig. 1a). The molecules possess a nearly planar extended π-conjugated system, with excellent π–π stacking between adjacent molecules, and with least-square mean plane distances of 3.265 Å and 3.253 Å (Fig. 1b). Overall, the crystal structure shows a close packing of the molecules with various van der Waals interactions in addition to ultra-close π-stacking interactions and S⋯S contacts of 3.374 Å.
1 ligand–metal complexes 1·Cu and 1·Zn·MeOH. 1H and 13C NMR spectra of complex 1·Zn·MeOH in DMSO-d6 revealed the presence of metal-coordinated MeOH molecules, suggesting a square pyramidal geometry around the metal center. It also indicated that the MeOH remained coordinated to the Zn even in the presence of DMSO. All attempts to grow single crystals of complex 1·Zn·MeOH, suitable for single crystal X-ray diffraction (SCXRD) analysis, were unsuccessful. Complex 1·Cu crystallizes with the acentric monoclinic space group C2/c. The complex has a square planar geometry around the metal center with a dihedral angle between the Cu–N(1)–O(2) and the Cu–N(2)–O(1) planes of 10.144(78)° (Fig. 1a). The molecules possess a nearly planar extended π-conjugated system, with excellent π–π stacking between adjacent molecules, and with least-square mean plane distances of 3.265 Å and 3.253 Å (Fig. 1b). Overall, the crystal structure shows a close packing of the molecules with various van der Waals interactions in addition to ultra-close π-stacking interactions and S⋯S contacts of 3.374 Å.
Cyclic voltammetry (CV) experiments were performed on 0.01 M solutions of 1, 1·Cu and 1·Zn·MeOH in dry DMF. In the anodic scans, 1, 1·Cu and 1·Zn·MeOH showed onset oxidation potentials (Eoxonset) of 0.65, 0.83 and 0.63 V (Fig. 1c), which correspond to HOMO energy levels of −5.05, −5.23 and −5.03 eV for 1, 1·Cu and 1·Zn·MeOH, respectively.9 The low-lying HOMOs indicate a good environmental stability of the ligand and the complexes. The higher HOMO level of 1·Zn·MeOH compared to that of 1·Cu may be attributed to the presence of the electron donating methanol molecule as an axial ligand.10 So, the HOMO energy level of 1·Zn·MeOH is closer to that of the work function potential of gold (−5.0 eV), than that of 1·Cu, by 0.2 eV. This, in turn, may facilitate the injection of holes from the gold electrodes to the active layers in transistors11 containing 1·Zn·MeOH, due to the decrease in the hole–injection barrier,12 compared to a device containing 1·Cu. All attempts to polymerize 1, 1·Cu and 1·Zn·MeOH electrochemically at their oxidation potentials in dry DMF were unsuccessful.
A detailed study of the transport measurements was performed on the ligand and the MOCs using a top-contact bottom gated field effect transistor (FET) architecture. The measurements indicated an excellent p-type transport with distinctive linear and saturation behaviors (Fig. 2). 1·Zn·MeOH and 1·Cu show μFET values as high as 1.5 and 0.7 cm2 V−1 s−1, respectively, with ON/OFF ratios >103. Though no annealing was performed on these spin coated films, the mobility values were three orders of magnitude greater than other solution processable metal–organic based molecules reported so far.13 Moreover, the mobility achieved was comparable to the best values of μFET obtained with similar MOCs from vacuum deposited devices.7 However, the FETs fabricated with 1 showed relatively low μFET values of 10−3–10−4 cm2 V−1 s−1. The insertion of metal atoms into the ligand framework enhanced the hole mobility by 3–4 orders of magnitude. This trend of an increase in μFET values with MOCs compared to the corresponding ligand can be related to the inherent crystallinities and efficient packing, with ultra-close π-stacking interactions. In both the square planar and the square pyramidal complexes, the metal ions force the ligand framework to adopt a planar geometry and, in turn, show intermolecular ultra-close π-stacking interactions. The higher mobilities of the devices containing 1·Zn·MeOH compared to those containing 1·Cu, are also in agreement with the observations that the polar square pyramidal complexes show better mobilities than the square planar complexes.7 The high mobility of the square pyramidal complex is expected due to the capability of strong π–π interactions between the concave and the convex pairs in such a complex.7a Monomeric Zn(II) square pyramidal complexes based on salphen derivatives have shown strong π–π stacking.14 Conductivity (σ) values obtained from the linear regime of the FET characteristics is given as 0.07–0.13 S cm−1 for 1·Cu which increases to 0.16–0.2 S cm−1 for 1·Zn·MeOH.
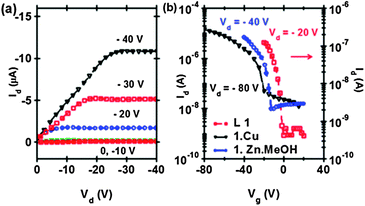 | ||
| Fig. 2 Typical (a) output curves for 1·Zn·MeOH and (b) transconductance curves for 1·Zn·MeOH, 1·Cu and ligand 1 in a FET geometry with W = 1 mm and L = 60–80 μm. | ||
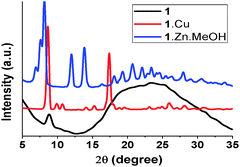
Thin films of 1, 1·Cu and 1·Zn·MeOH for X-ray diffraction (XRD) studies were prepared using the same procedure used for the device fabrication. The XRD measurements obtained from the thin film of ligand 1 show predominantly amorphous features, whereas the complexes show crystalline peaks in the thin film XRD analysis with inter-planar distances of around 3.1 Å for 1·Cu (2θ = 25.88), and around 2.9 Å for 1·Zn·MeOH (2θ = 27.96) using a Cu-Kβ filter (Fig. 3). The inter-planar distances calculated from the thin film XRD analysis of 1·Cu were comparable to those from SCXRD analysis. This magnitude of inter-planar distance is comparatively smaller than for other organic molecules, hence the enhanced overlap of π-electrons is expected in these MOCs, which can support disorder free 2D transport (Fig. 1b). Furthermore, the thin film and powder XRD patterns of 1·Zn·MeOH were found to be similar (Fig. S5, ESI†), indicating identical coordination around the metal center. The SEM image of the thin film of complex 1·Cu also revealed its highly crystalline nature, with micro-crystals of around 20 μm, while that of 1·Zn·MeOH showed crystallites with particle sizes of around 5 μm (Fig. S7, ESI†).
A metal atom attached to a conjugated moiety can modify the transport behavior of a complex, if the metal–organic combination is redox-active.6a,b It was observed that μMOCFET > μligandFET. A clearer picture of the role of the metal atom has evolved upon comparing the MOCs based on Cu and Zn. The mobility of 1·Cu was observed to be smaller than that of 1·Zn·MeOH. Probably, the lower electronegativity of zinc compared to copper, and the presence of an electron donating group in complex 1·Zn·MeOH, can facilitate the hole formation/stabilization, and thus is responsible for the observed trends in the mobilities of these molecules. The CV measurements also demonstrate the ease of oxidizing 1·Zn·MeOH compared to 1·Cu, validating the role of the metal atom and the additional donor (methanol) in modifying the electronic structure of these complexes. We define the parameter D = dσ/dVg (where Vg is the gate bias) which is a measure of the “ease of doping” with an external bias. The magnitude of D, estimated from the output characteristics, turns out to be 4.5 × 10−3 S cm−1 V−1 for 1·Zn·MeOH which is three time greater than for 1·Cu, clearly demonstrating the role of the metal atom in modifying the donor properties.
In summary, a new thiophene-based salphen-type ligand 1, and its Cu(II) and Zn(II) complexes, were synthesized in high yields. The planar delocalized π-electron systems in the MOCs resulted in ultra-close π-stacking interactions. In turn, the MOCs exhibited excellent p-type mobilities using solution processed thin films (as high as 0.7 and 1.5 cm2 V−1 s−1 for 1·Cu and 1·Zn·MeOH, respectively). The incorporation of a metal ion in a conjugated ligand framework, and the geometries of the resulting MOCs, played important roles in tuning the performances of the OFET devices.
SP acknowledges DST for funding (Fast-Track Project: SR/FT/CS-17/2011).
Notes and references
- (a) J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, J. Am. Chem. Soc., 2013, 135, 6724 CrossRef CAS PubMed; (b) G. C. Papavassiliou, G. C. Anyfantis and G. A. Mousdis, Crystals, 2012, 2, 762 CrossRef CAS PubMed; (c) B. J. Jung, N. J. Tremblay, M.-L. Yeh and H. E. Katz, Chem. Mater., 2011, 23, 568 CrossRef CAS; (d) D. Natali and M. Caironi, Adv. Mater., 2012, 24, 1357 CrossRef CAS PubMed; (e) C.-L. Ho and W.-Y. Wong, Coord. Chem. Rev., 2011, 255, 2469 CrossRef CAS PubMed; (f) L. Li, Q. Tang, H. Li, W. Hu, X. Yang, Z. Shuai, Y. Liu and D. Zhu, Pure Appl. Chem., 2008, 80, 2231 CAS; (g) V. Coropceanu, J. Cornil, D. A. d. S. Filho, Y. Olivier, R. Silbey and J.-L. Bredas, Chem. Rev., 2007, 107, 926 CrossRef CAS PubMed.
- (a) M. M. Torrent and C. Rovira, Chem. Rev., 2011, 111, 4833 CrossRef PubMed; (b) M. D. Curtis, J. Cao and J. W. Kampf, J. Am. Chem. Soc., 2004, 126, 4318 CrossRef CAS PubMed.
- (a) K. C. Gupta and A. K. Sutar, Coord. Chem. Rev., 2008, 252, 1420 CrossRef CAS PubMed; (b) A. Tzubery and E. Y. Tshuva, Inorg. Chem., 2011, 50, 7946 CrossRef CAS PubMed; (c) S. J. Wezenberg and A. W. Kleij, Angew. Chem., Int. Ed., 2008, 47, 2354 CrossRef CAS PubMed; (d) H. Miyasaka, A. Saitoh and S. Abe, Coord. Chem. Rev., 2007, 251, 2622 CrossRef CAS PubMed; (e) T. Glaser, Chem. Commun., 2011, 47, 116 RSC; (f) L. D. Chen, D. Mandal, G. Pozzi, J. A. Gladysz and P. Bühlmann, J. Am. Chem. Soc., 2011, 133, 20869 CrossRef CAS PubMed.
- (a) S. Yamada, Coord. Chem. Rev., 1999, 190–192, 537 CrossRef CAS; (b) C. J. Whiteoak, G. Salassa and A. W. Kleij, Chem. Soc. Rev., 2012, 41, 622 RSC; (c) J. Lewiński, J. Zachara, I. Justyniak and M. Dranka, Coord. Chem. Rev., 2005, 249, 1185 CrossRef PubMed; (d) M. Kojima, H. Taguchi, M. Tsuchimoto and K. Nakajima, Coord. Chem. Rev., 2003, 237, 183 CrossRef CAS.
- (a) A. Zulauf, X. Hong, F. Brisset, E. Schulz and M. Mellah, New J. Chem., 2012, 36, 1399 RSC; (b) A. Pietrangelo, B. C. Sih, B. N. Boden, Z. Wang, Q. Li, K. C. Chou, M. J. MacLachlan and M. O. Wolf, Adv. Mater., 2008, 20, 2280 CrossRef CAS; (c) R. P. Kingsborough and T. M. Swager, J. Am. Chem. Soc., 1999, 121, 8825 CrossRef CAS; (d) R. P. Kingsborough and T. M. Swager, Adv. Mater., 1998, 10, 1100 CrossRef CAS.
- (a) Z. Bao, A. J. Lovinger and A. Dodabalapur, Appl. Phys. Lett., 1996, 69, 3066 CrossRef CAS PubMed; (b) Z. Bao, A. J. Lovinger and A. Dodabalapur, Adv. Mater., 1997, 9, 42 CrossRef CAS; (c) T. D. Anthopoulos, S. Setayesh, E. Smits, M. Colle, E. Cantatore, B. de Boer, P. W. M. Blom and D. M. de Leeuw, Adv. Mater., 2006, 18, 1900 CrossRef CAS; (d) E. C. P. Smits, T. D. Anthopoulos, S. Setayesh, E. van Veenendaal, R. Coehoorn, P. W. M. Blom, B. de Boer and D. M. de Leeuw, Phys. Rev., 2006, 73, 205316 CrossRef; (e) T. Taguchi, H. Wada, T. Kambayashi, B. Noda, M. Goto, T. Mori, K. Ishikawa and H. Takezoe, Chem. Phys. Lett., 2006, 421, 395 CrossRef CAS PubMed; (f) T. D. Anthopoulos, G. C. Anyfantis, G. C. Papavassiliou and D. M. de Leeuw, Appl. Phys. Lett., 2007, 90, 122105 CrossRef PubMed; (g) J. Y. Cho, B. Domercq, S. C. Jones, J. Yu, X. Zhang, Z. An, M. Bishop, S. Barlow, S. R. Marder and B. Kippelen, J. Mater. Chem., 2007, 17, 2642 RSC; (h) G. C. Papavassiliou, G. C. Anyfantis, C. P. Raptopoulou, V. Psycharis, N. Ioannidis, V. Petrouleas and P. Paraskevopoulou, Polyhedron, 2009, 28, 3368 CrossRef CAS PubMed; (i) H. Wada, T. Taguchi, B. Noda, T. Kambayashi, T. Mori, K. Ishikawa and H. Takezoe, Org. Electron., 2007, 8, 759 CrossRef CAS PubMed; (j) S. Dalgleish, H. Yoshikawa, M. M. Matsushita, K. Awaga and N. Robertson, Chem. Sci., 2011, 2, 316 RSC; (k) S. Dalgleish, J. G. Labram, Z. Li, J. P. Wang, C. R. Mcneill, T. D. Anthopoulos, N. C. Greenham and N. Robertson, J. Mater. Chem., 2011, 21, 15422 RSC; (l) J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, J. Am. Chem. Soc., 2013, 135, 6724 CrossRef CAS PubMed; (m) L. Qu, Y. Guo, H. Luo, C. Zhong, G. Yu, Y. Liu and J. Qin, Chem. Commun., 2012, 48, 9965 RSC; (n) C. Pearson, A. J. Moore, J. E. Gibson, M. R. Bryce and M. C. Petty, Thin Solid Films, 1994, 244, 932 CrossRef CAS.
- (a) L. Li, Q. Tang, H. Li, X. Yang, W. Hu, Y. Song, Z. Shuai, W. Xu, Y. Liu and D. Zhu, Adv. Mater., 2007, 19, 2613 CrossRef CAS; (b) H. Wang, D. Song, J. Yang, B. Yu, Y. Geng and D. Yan, Appl. Phys. Lett., 2007, 90, 253510 CrossRef PubMed.
- A. Pietrangelo, B. N. Boden, M. J. MacLachlan and M. O. Wolf, Can. J. Chem., 2009, 87, 314 CrossRef CAS.
- (a) A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2nd edn, 2001 Search PubMed; (b) HOMO levels were calculated using, EHomo = −(4.8 − E1/2ferrocene + Eoxonset). E1/2ferrocene was calculated to be 0.4 V in DMF–TBAPC system.
- X. Cai, Y. Zhang, D. Qi and J. Jiang, J. Phys. Chem. A, 2009, 113, 2500 CrossRef CAS PubMed.
- U. Purushotham and G. N. Sastry, Phys. Chem. Chem. Phys., 2013, 15, 5039 RSC.
- N. Kawasaki, Y. Ohta, Y. Kubozono and A. Fujiwara, Appl. Phys. Lett., 2007, 91, 123518 CrossRef PubMed.
- L. Li, Q. Tang, H. Li, W. Hu, X. Yang, Z. Shuai, Y. Liu and D. Zhu, Pure Appl. Chem., 2008, 80, 2231 CAS.
- (a) A. W. Kleij, M. Kuil, M. Lutz, D. M. Tooke, A. L. Spek, P. C. J. Kamer, P. W. N. M. van Leeuwen and J. N. H. Reek, Inorg. Chim. Acta, 2006, 359, 1807 CrossRef CAS PubMed; (b) N. E. Eltayeb, S. G. Teoh, J. B.-J. Teh, H.-K. Fun and K. Ibrahim, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m1764 CAS; (c) E. C. Escudero-Adán, J. Benet-Buchholz and A. W. Kleij, Chem. – Eur. J., 2009, 15, 4233 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization data and device fabrication methods. CCDC 920697 and 920799. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4cc01360c |
| This journal is © The Royal Society of Chemistry 2014 |