Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
NMR methodology for complex mixture ‘separation’†
Nicholle G. A.
Bell
a,
Lorna
Murray
a,
Margaret C.
Graham
b and
Dušan
Uhrín
*a
aEastChem, School of Chemistry, University of Edinburgh, King's Buildings, West Mains Rd, Edinburgh, Scotland EH9 3JJ, UK. E-mail: dusan.uhrin@ed.ac.uk
bSchool of Geosciences, University of Edinburgh, King's Buildings, West Mains Rd, Edinburgh, Scotland EH9 3JN, UK
First published on 2nd January 2014
Abstract
Mixture ‘separation’ by NMR is demonstrated through the development of a pseudo 4D NMR experiment, 3D IPAP INEPT-INADEQUATE-HSQC, designed for the structural elucidation of 13C tagged compounds.
The structural elucidation of compounds contained within complex mixtures is a challenging task. Despite the advances in chromatography, some mixtures cannot be separated. Humic substances (HS), produced by the biodegradation of plant and animal residues,1 are the best known example of an ‘inseparable’ mixture consisting of thousands of organic compounds. Ubiquitous in nature, they make up a considerable proportion of the Earth's carbon pool and are key players in many biogeochemical processes.
In order to comprehend the functional roles of HS on a molecular level, their structural composition needs to be deciphered. So far HS have been characterized only on the level of compound classes and presence of individual functional groups.2–4 NMR spectroscopy and mass spectrometry are widely regarded as the two most promising analytical techniques for revealing the structure of individual HS molecules.5–7 Nevertheless, HS pose a considerable analytical challenge to both techniques, e.g., the signal overlap in NMR spectra of complex mixtures prevents separation of resonances belonging to individual molecules and hence their identification using standard NMR techniques. Increasing the dimensionality of NMR experiments, selective excitation, or DOSY spectroscopy8,9 are the three most common approaches attempted to circumvent these problems.
Unfortunately, these quickly fail when the complexity of mixtures increases. Inevitably, for chromatographically inseparable mixtures the “separation” must be done spectroscopically. One way of achieving this is by tagging the molecules with isotopically labelled nuclei. Once in place, the polarization transfer pathways are directed through these tags, reducing the complexity of spectra significantly. As illustrated here, this approach has a potential to elucidate molecular fragments of compounds contained in complex mixtures.
The bulk of HS is composed of oxygen rich HxCyOz compounds with the Mw range of ∼200–1000 g mol−1.10 Particularly prevalent functionalities decorating aromatic and aliphatic skeletons of HS are the hydroxyl and carboxyl groups. These groups are crucial to the interaction of HS with species such as heavy metals as well as the self-association of HS molecules. The methodology presented here aims at characterizing the aromatic moieties of HS carrying OH and COOH groups and relies on introducing 13C-enriched –O13CH3 and –COO13CH3 groups into HS compounds. HS have been methylated in the past and the inspection of –O13CH3 resonances yielded some rudimentary information about the nature of their COOH and OH groups.11 The novelty of our approach is that it uses labels to spy on their neighbourhood, obtaining the 1H and 13C chemical shifts and 1H–1H and 1H–13C coupling constants of the nuclei in their vicinity.
To achieve this aim we have designed a novel 3D NMR experiments, referred to here as 3D IPAP INEPT-INADEQUATE-HSQC (Fig. 1).
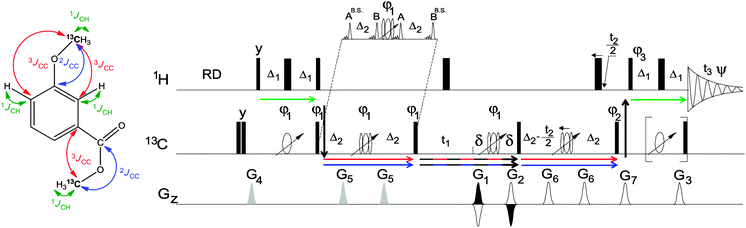 | ||
| Fig. 1 The pulse sequence of the 3D IPAP INEPT-INADEQUATE-HSQC and its polarisation transfer pathways utilising various coupling constants. The details of the pulse sequence are given in the ESI.† | ||
This NMR experiment can be viewed as a 3D extension of a 2D INEPT-INADEQUATE.12 It is also related to 3D HCCH experiments.13,14 3D IPAP INEPT-INADEQUATE-HSQC exploits polarization transfer pathways shown in Fig. 1, and correlates the double-quantum (DQ) coherencies of long-range coupled carbons (F1) with corresponding single-quantum 13C chemical shifts (F2) and the 1H chemical shifts (F3). The polarizations transfer is tuned for 2,3JCC (Fig. S1, ESI†) and correlates the chemical shifts of nuclei in 13CH3⋯13CHy (y = 0, 1) fragments. It starts and ends concurrently on methoxy and aromatic protons located next to the methoxy groups. For CH3⋯CH fragments it provides chemical shifts of all four nuclei and can therefore be regarded as a pseudo 4D experiment; all three chemical shifts are obtained for the CH3⋯Cq moieties. Since the acquired NMR signal is filtered via isotopically enriched 13CH3 groups, the resulting spectra are significantly simplified.
The limited 1H and 13C chemical shifts range of methoxy groups (Fig. S2, ESI†) necessitates the use of high digital resolution in the F2 and F3 dimensions of the 3D spectra. In order to achieve this in a realistic time, we have (i) optimized 1H and 13C carrier frequencies and spectral widths in all dimensions, (ii) frequency shifted DQ coherences during t1, (iii) aliased signals in the F2 dimension, (iv) used non-uniform sampling and (v) acquired pure in phase and antiphase (IPAP)1513C-coupled multiplets during t3, as detailed in the ESI,† Fig. S3–S5.
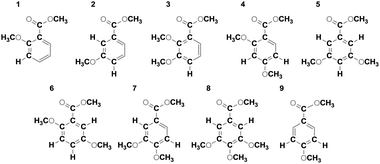
The proposed method is illustrated on a model mixture of nine benzoic acid derivatives, 1a–9a, which were fully methylated using 13CH3I, yielding compounds 1–9 (Scheme 1, average concentration of 1.4 mM).
2D F2F3 (HSQC) (Fig. 2a and b) and 2D F1F3 (DQ) (Fig. 2c and d) planes of the 3D IPAP INEPT-INADEQUATE-HSQC spectrum of the model mixture illustrate the resolving power of this experiment. The spectrum was analysed as outlined in Fig. 2e–g. Shown here are the 2D DQ (F1F3) planes extracted at the 13C shifts of the aromatic and Me carbons (F2) indicated by red arrows in Fig. 2a and b. The obtained 13C and 1H chemical shifts allowed unambiguous identification of the fragment highlighted in the inset. The fragment was assigned to the correct molecule by considering the electronic effects of OMe and COOMe groups on the 13C and 1H chemical shifts of benzene and by analysing the proton–proton and long-range proton–carbon coupling constants determined in F3. The inspection of the complete 3D spectrum lead to the identification of the fragments highlighted in Scheme 1 and their assignment to individual molecules.
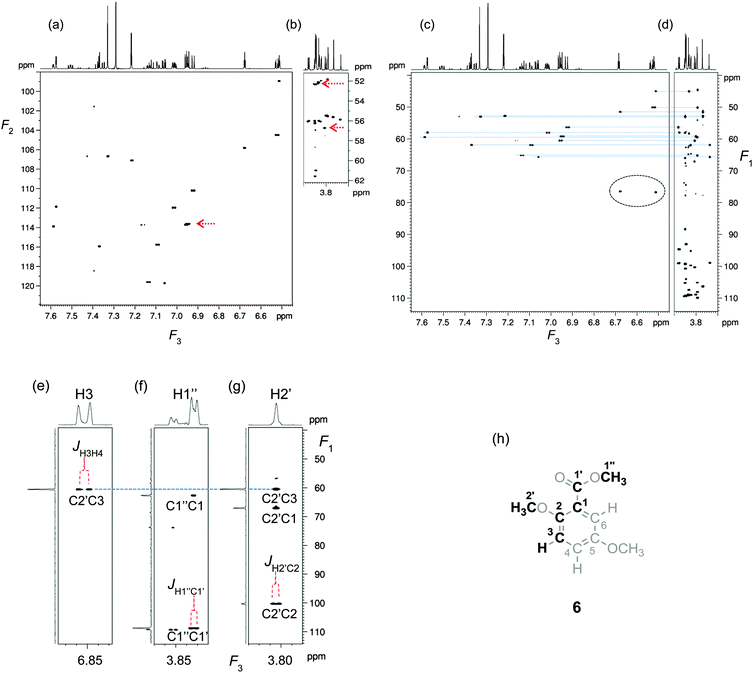 | ||
| Fig. 2 2D projections of the 3D IPAP INEPT-INADEQUATE-HSQC spectrum obtained by the addition of the inphase and anti-phase 3D spectra. (a, b) F2F3 HSQC and (c, d) F1F3 DQ projections of the aromatic (a, c) and methyl (b, d) regions of the spectrum. The circled signals belong to the aliased DQ coherences of OMe carbons in compounds 4 and 5. See Fig. S4 (ESI†) for the explanation of their origin. Blue lines connect DQ coherences of CH3⋯CH fragments detected simultaneously on the aromatic and methyl protons. The red arrow indicates the F2 coordinates of planes shown in (e–g). 2D DQ (F1F3) planes extracted at the CH and OMe 13C chemical shifts indicated in (a and b) showing (e) aromatic, (f) ester and (g) methoxy proton regions of the spectrum. The blue line indicates a shared DQ frequency. Observed splittings are annotated. (h) The identified molecular fragment. | ||
When applied to HS, the signals from methylated aliphatic hydroxyl and carboxyl groups can potentially interfere with the interpretation of the 3D IPAP INEPT-INADEQUATE-HSQC spectra. These can be eliminated by band-selective inversion of spins resonating within the 65–95 and 15–45 ppm regions of the 13C spectra applied during the initial carbon spin-echo, 2Δ2, (see the inset of Fig. 1). Spins resonating in these regions effectively receive a 360° pulse, thus refocusing their 2,3JCC couplings with the 13CH3O carbons and eliminating their signals (see Fig. S6, ESI†).
How sensitive is the 3D IPAP INEPT-INADEQUATE-HSQC experiment? Because the DQ coherences are created between the fully labelled 13CH3 carbons and natural abundance 13C spins, the theoretical sensitivity of this experiment is comparable to that of a refocused gradient-selected 1H, 13C HMBC optimized for nJCH couplings. We have determined the S/N ratios in 1D F3 traces extracted from F1F3 planes of the 3D spectrum obtained by the addition of the 3D IP and AP INEPT-INADEQUATE-HSQC spectra. The values were normalized (Fig. S7, ESI†) to the average concentration of the methylated compounds (1.4 mM). Due to their singlet character, the S/N is higher for –O13CH3 (60–1900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) than for the aromatic resonances (57–600
1) than for the aromatic resonances (57–600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Somewhat lower values were obtained for –COO13CH3 signals (40–240
1). Somewhat lower values were obtained for –COO13CH3 signals (40–240![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These values clearly reflect the sizes of 2,3JCC couplings (Fig. S1, ESI†) involved in the polarisation transfer and can be increased for small coupling constants by optimizing the Δ2 intervals for smaller JCC couplings (6 Hz at present). This will have to be balanced against faster relaxation in larger molecules. We note that the relaxation effects can be reduced by using 13CD2H in place of 13CH3 groups. This modification opens a route toward a design of more efficient 3D INADEQUATE-based experiments thus compensating partially for the loss of protons in 3CD2H groups.
1). These values clearly reflect the sizes of 2,3JCC couplings (Fig. S1, ESI†) involved in the polarisation transfer and can be increased for small coupling constants by optimizing the Δ2 intervals for smaller JCC couplings (6 Hz at present). This will have to be balanced against faster relaxation in larger molecules. We note that the relaxation effects can be reduced by using 13CD2H in place of 13CH3 groups. This modification opens a route toward a design of more efficient 3D INADEQUATE-based experiments thus compensating partially for the loss of protons in 3CD2H groups.
Considering the lowest S/N observed (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a 10-fold reduction of the concentration would still yield spectra of adequate quality. Thus assuming a 140 μM concentration of a 500 g mol−1Mw compound, 38.5 μg dissolved in 550 μl of CDCl3 are required per compound. A 19.3 mg strong mixture of compounds containing 500 unique substitution patterns by –OH and –COOH groups on aromatic rings will therefore provide 3D spectra with sufficient S/N ratios to be analyzed. As the methylation procedure includes extraction into the organic phase, and thus selects a subset of HS compounds, simplification of these complex mixtures is inherent to this process. This could be further improved by employing partial fractionation of HS prior to methylation.
1), a 10-fold reduction of the concentration would still yield spectra of adequate quality. Thus assuming a 140 μM concentration of a 500 g mol−1Mw compound, 38.5 μg dissolved in 550 μl of CDCl3 are required per compound. A 19.3 mg strong mixture of compounds containing 500 unique substitution patterns by –OH and –COOH groups on aromatic rings will therefore provide 3D spectra with sufficient S/N ratios to be analyzed. As the methylation procedure includes extraction into the organic phase, and thus selects a subset of HS compounds, simplification of these complex mixtures is inherent to this process. This could be further improved by employing partial fractionation of HS prior to methylation.
Obtaining 1H and 13C chemical shifts of the nuclei in the vicinity OH and COOH by itself does not lead to a structure, and such data needs to be interpreted by considering the precursors of HS molecules and their chemical shifts. However, it is clear that this methodology supersedes information content of simple 1H, 13C HSQC spectra of HS.16 The key is the increased number of nuclei that are identified as belonging to the same molecule. Additional valuable information can be obtained by hydrolysing the –COO13CH3 esters, while keeping the –O13CH3 groups untouched. The resulting site-specific changes of chemical shifts of aromatic spins can be quantified through recording the 3D IPAP INEPT-INADEQUATE-HSQC of a partially hydrolysed mixture (data not shown). Such information is very valuable for the identification of fragments carrying both OH and COOH groups.
In conclusion, we conjecture that tagging by 13C labelled methyl groups in combination with 3D NMR spectroscopy is a very promising approach to the analysis of complex mixtures such as HS. To the best of our knowledge, this work is the first example of a sophisticated NMR experiment designed to explore the chemical environment around the tags, rather than just the signals from the tags themselves. This approach is not limited to methylation, other tags, containing NMR active, fully abundant nuclei such as 15N or 19F can also be explored. Finally, many other mixtures can benefit from this approach, including those found in food, beverages, natural products, or biological samples, to name a few.
This work was supported by the NERC grant NE/L00044X/1 to MCG and DU. NGAB would like to acknowledge the support of the University of Edinburgh Principal's Career Development Scholarship and NERC. We thank J. Bella for maintaining the NMR spectrometers.
Notes and references
- F. J. Stevenson, Humus Chemistry: Genesis, Composition, Reactions, John Wiley & Sons, Ltd, New York, 2nd edn, 1994 Search PubMed.
- B. Lam, A. Baer, M. Alaee, B. Lefebvre, A. Moser, A. Williams and A. J. Simpson, Environ. Sci. Technol., 2007, 41, 8240–8247 CrossRef CAS.
- N. Hertkorn, R. Benner, M. Frommberger, P. Schmitt-Kopplin, M. Witt, K. Kaiser, A. Kettrup and J. I. Hedges, Geochim. Cosmochim. Acta, 2006, 70, 2990–3010 CrossRef CAS PubMed.
- R. Benner, J. D. Pakulski, M. McCarthy, J. I. Hedges and P. G. Hatcher, Science, 1992, 255, 1561–1564 CAS.
- L. A. Cardoza, A. K. Korir, W. H. Otto, C. J. Wurrey and C. K. Larive, Prog. Nucl. Magn. Reson. Spectrosc., 2004, 45, 209–238 CrossRef CAS PubMed.
- N. Hertkorn, C. Ruecker, M. Meringer, R. Gugisch, M. Frommberger, E. M. Perdue, M. Witt and P. Schmitt-Kopplin, Anal. Bioanal. Chem., 2007, 389, 1311–1327 CrossRef CAS PubMed.
- A. J. Simpson, D. J. McNally and M. J. Simpson, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 58, 97–175 CrossRef CAS PubMed.
- A. J. Simpson, W. L. Kingery and P. G. Hatcher, Environ. Sci. Technol., 2003, 37, 337–342 CrossRef CAS.
- R. Novoa-Carballal, E. Fernandez-Megia, C. Jimenez and R. Riguera, Nat. Prod. Rep., 2011, 28, 78–98 RSC.
- A. C. Stenson, W. M. Landing, A. G. Marshall and W. T. Cooper, Anal. Chem., 2002, 74, 4397–4409 CrossRef CAS.
- M. A. Mikita, C. Steelink and R. L. Wershaw, Anal. Chem., 1981, 53, 1715–1717 CrossRef CAS.
- J. Weigelt and G. Otting, J. Magn. Reson., Ser. A, 1995, 113, 128–130 CrossRef CAS.
- J. Chung, J. R. Tolman, K. P. Howard and J. H. Prestegard, J. Magn. Reson., Ser. B, 1993, 102, 137–147 CrossRef CAS.
- T. Saito and R. L. Rinaldi, J. Magn. Reson., Ser. A, 1996, 118, 136–139 CrossRef CAS.
- L. S. Yao, J. F. Ying and A. Bax, J. Biomol. NMR, 2009, 43, 161–170 CrossRef CAS PubMed.
- E. M. Perdue, N. Hertkorn and A. Kettrup, Anal. Chem., 2007, 79, 1010–1021 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, details of the optimization of NMR parameters, values of coupling constants, S/N ratios, examples of processing of IPAP spectra and a suppression of signals from aliphatic OMe groups. See DOI: 10.1039/c3cc48907h |
| This journal is © The Royal Society of Chemistry 2014 |