Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Historical textile dyeing with Genista tinctoria L.: a comprehensive study by UPLC-MS/MS analysis†
Lore G.
Troalen
ab,
Ashley S.
Phillips
ac,
David A.
Peggie
ad,
Perdita E.
Barran
ac and
Alison N.
Hulme
*a
aEaStCHEM School of Chemistry, Joseph Black Building, Kings Buildings, West Mains Road, Edinburgh, EH9 3JJ, UK. E-mail: Alison.Hulme@ed.ac.uk
bNational Museums Scotland, Collections Services Department, 242 West Granton Road, Edinburgh, EH5 1JA, UK
cManchester Institute of Biotechnology and School of Chemistry, The University of Manchester, 131 Princess Street, Manchester M1 7DN, UK
dNational Gallery London, Scientific Department, Trafalgar Square, London WC2N 5DN, UK
First published on 22nd July 2014
Abstract
Polyphenolic components from Genista species have been well characterised because of their potential as antioxidants and as therapeutic leads; however, the identification of dyer's greenweed (Genista tinctoria L.) in historical textiles has been the subject of only limited studies. This paper presents a comprehensive UPLC-PDA MS/MS study of reference and historical yarns dyed with this species. Several so far unreported dye components that could assist with the identification of this dye source, were characterised by MS/MS. Furthermore, the effect of photo-degradation and textile preparation techniques (such as over-dyeing) on the dye fingerprint was investigated and the results correlated with those obtained from historical samples from the Burrell and Bodleian collections, UK.
1. Introduction
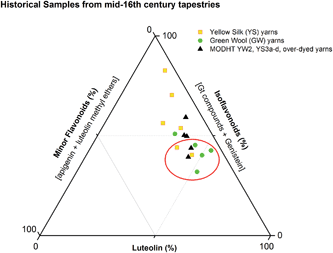
Polyphenolic components from Genista species have been well characterised because of their potential as antioxidants and as therapeutic leads;1,2 however, the identification of dyer's greenweed (Genista tinctoria L.) in historical textiles has been the subject of only limited studies.3,4 Although historically weld (Reseda luteola L.) was probably the most widely used European yellow dye plant, it is reported that other dye plants, including dyer's greenweed (Genista tinctoria L.) and sawwort (Serratula tinctoria L.), were used as substitutes.5 The flavones luteolin and apigenin, the isoflavone genistein, and the glycosides of these are known dye components of Genista species.6 But genistein, and its glycosides, are also the main dye components found in other varieties of broom; notwithstanding, the dye source for historical textiles is usually ascribed to dyer's greenweed.7,8 Whilst genistein is the principle component on which this attribution is made,4 our studies of the dye extracts of historical samples show the presence of additional dye components that could enhance this identification. This paper presents a comprehensive study of the structure of these additional dye components using UPLC-PDA‡ and ESI-MS/MS techniques. To achieve this, an analytical method was developed for a range of flavonoid and isoflavonoid dyes, allowing a more efficient separation of several isomeric dye components from textile samples. This method was then used to determine the relative amount of dyestuffs present in raw materials, modern yarns and historical yarns; examining differences between the plant extract and the dye components adsorbed onto textiles, and relating variations in component ratios to dyeing processes such as over-dyeing, and to the effects of photo-degradation. Data from a selection of historical yarns sampled from mid-sixteenth century English tapestries from the Burrell Collection in Glasgow, UK and the Bodleian Library in Oxford, UK were then placed in context using the results of this study.2. Experimental
2.1 Materials and chemicals
An authentic sample of isoprunetin (4′,7-dihydroxy-5-methoxyisoflavone) was prepared from genistein by selective acetylation (Ac2O, py) to give 7,4′-diacetoxy-5-hydroxyisoflavone,10 methylation of the remaining free hydroxyl [(MeO)2SO2 K2CO3, acetone] under high dilution conditions11 to give 7,4′-diacetoxy-5-methoxyisoflavone, followed by hydrolysis (NaHCO3 aq., MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF)10 of the acetate groups which allowed isolation of isoprunetin in >95% purity after chromatography (see ESI 1†).12
THF)10 of the acetate groups which allowed isolation of isoprunetin in >95% purity after chromatography (see ESI 1†).12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [25 mL, 1
MeOH [25 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v); equivalent to 40 μg mL−1]; (2) a solution containing apigenin, chrysoeriol (0.20 ± 0.01 mg of each standard) in H2O
1 (v/v); equivalent to 40 μg mL−1]; (2) a solution containing apigenin, chrysoeriol (0.20 ± 0.01 mg of each standard) in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [10 mL, 1
MeOH [10 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v); equivalent to 20 μg mL−1]; (3) a solution of diosmetin (1.00 ± 0.01 mg) in H2O
1 (v/v); equivalent to 20 μg mL−1]; (3) a solution of diosmetin (1.00 ± 0.01 mg) in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [25 mL, 1
MeOH [25 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v); equivalent to 40 μg mL−1]. Diluted solutions were then prepared with components at concentrations of 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.02 and 0.01 μg mL−1, by dilution with H2O
1 (v/v); equivalent to 40 μg mL−1]. Diluted solutions were then prepared with components at concentrations of 20, 10, 5, 1, 0.5, 0.1, 0.05, 0.02 and 0.01 μg mL−1, by dilution with H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [1
MeOH [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)] using calibrated micro-pipettes.
1 (v/v)] using calibrated micro-pipettes.
2.2 UPLC-PDA and ESI MS systems
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23B) then a linear gradient from 3.33 min to 29.33 min (10A
23B) then a linear gradient from 3.33 min to 29.33 min (10A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90B) before recovery of the initial conditions over 1 min and equilibration over 7 min.
90B) before recovery of the initial conditions over 1 min and equilibration over 7 min.
2.3 Plant and yarn extractions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in an ultrasonic bath for 2 hours at 40 °C. A fraction of the extract was centrifuged for 10 min at 10
1, v/v) in an ultrasonic bath for 2 hours at 40 °C. A fraction of the extract was centrifuged for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and filtered using a PTFE Phenomenex syringe filter (0.2 μm, 4 mm) for UPLC-PDA analysis. A second fraction of the extract was subjected to the hydrolysis procedure described below for yarn analysis and again analysed by UPLC-PDA.
000 rpm and filtered using a PTFE Phenomenex syringe filter (0.2 μm, 4 mm) for UPLC-PDA analysis. A second fraction of the extract was subjected to the hydrolysis procedure described below for yarn analysis and again analysed by UPLC-PDA.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [200 μL, 2
MeOH [200 μL, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v/v)], at 100 °C for 10 min.13 After ambient cooling to room temperature, the extract was centrifuged for 10 min at 10
1 (v/v/v)], at 100 °C for 10 min.13 After ambient cooling to room temperature, the extract was centrifuged for 10 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and then filtered directly into Waters UPLC vials® (residual volume of 9 μL) using a PTFE Phenomenex syringe filter (0.2 μm, 4 mm). The extract was then cooled with liquid nitrogen and dried under vacuum using a freeze drier system. The dry residue was then reconstituted with H2O
000 rpm and then filtered directly into Waters UPLC vials® (residual volume of 9 μL) using a PTFE Phenomenex syringe filter (0.2 μm, 4 mm). The extract was then cooled with liquid nitrogen and dried under vacuum using a freeze drier system. The dry residue was then reconstituted with H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH [40 μL, 1
MeOH [40 μL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)] – allowing a single injection of 10 μL.
1 (v/v)] – allowing a single injection of 10 μL.
2.4 Accelerated light-ageing system
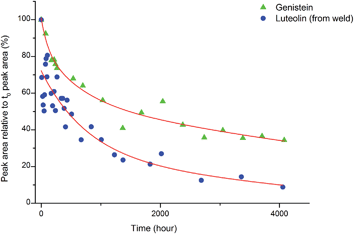
Accelerated light-ageing experiments were performed at National Museums Scotland using a Complete Lighting Systems light box (St Albans, UK), working at ambient temperature and relative humidity (RH). These levels were monitored hourly, together with the intensity of illuminance and UV levels using an Elsec IRLOG environmental Logger and the data periodically downloaded to computer. Wool yarns dyed with weld (Reseda luteola L.) and others with the single dye component genistein were removed over a period of 4571 hours and 1 mg of yarn was subsequently hydrolysed following the procedure described above and investigated by HPLC analysis following the method used by Peggie et al. in order to obtain the degradation profiles of luteolin and genistein components.143. Results and discussion
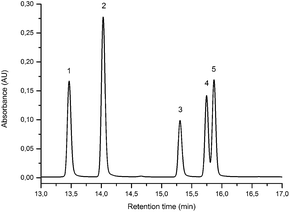
3.1 UPLC and MS study of flavone/isoflavone standards
| Component | Structure | PDA Rt (min) | LoD (LoQ) (ng) | λ max MeOH (nm) | MS/MS Rt (min) | [M − H]−m/z | MS/MS (CE: 25 eV) m/z (% BPI) fragment | |
|---|---|---|---|---|---|---|---|---|
| a The ions annotated with RDA correspond to retro-Diels–Alder fragments (1,3A−). The ions annotated with RDA* correspond to retro-Diels–Alder fragments where ionisation is in the B-ring (1,3B−). The ions annotated with iFF correspond to the isoflavonoid-specific fragmentation (0,3B−). | ||||||||
| FLAVONES | ||||||||
| Luteolin (1) |
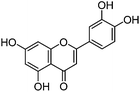
|
13.47 | 0.9 (2.6) | 13.56 | 285 | 285 (100) | [M − H]− | |
| 217 (12.5) | [M − H − C3O2]− | |||||||
| 199 (17.8) | ||||||||
| 252 | 175 (23.1) | [M − H − C3O2 − C2H2O]− | ||||||
| 291 (sh) | 151 (45.2) | RDA | ||||||
| 349 | 149 (20.2) | (4′-OH) | ||||||
| 133 (63.5) | RDA* | |||||||
| 121 (6.6) | (4′-OH) − CO | |||||||
| 107 (12.1) | RDA − CO2 | |||||||
| Apigenin (3) |
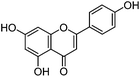
|
15.26 | 1.9 (5.7) | 15.58 | 269 | 269 (100) | [M − H]− | |
| 225 (28.5) | [M − H − CO2]− | |||||||
| 201 (12.9) | [M − H − C3O2]− | |||||||
| 267 | 183 (9.5) | |||||||
| 300 (sh) | 159 (12.6) | [M − H − C3O2 − C2H2O]− | ||||||
| 338 | 151 (58.7) | RDA | ||||||
| 149 (54.9) | (4′-OH) | |||||||
| 121 (11.3) | (4′-OH) − CO | |||||||
| 117 (79.1) | RDA* | |||||||
| 107 (17.4) | RDA − CO2 | |||||||
| Chrysoeriol (4) |
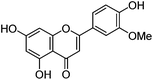
|
15.69 | 1.2 (3.4) | 250 | 15.80 | 299 | 299 (4.4) | [M − H]− |
| 268 | 284 (100) | [M − H − CH3]−˙ | ||||||
| 290 (sh) | 256 (30.1) | [M − H − CH3 − CO]−˙ | ||||||
| 349 | 151 (1.3) | |||||||
| Diosmetin (5) |
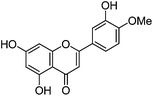
|
15.87 | 1.2 (3.7) | 250 | 15.93 | 299 | 299 (1.3) | [M − H]− |
| 268 | 284 (100) | [M − H − CH3]−˙ | ||||||
| 290 (sh) | 256 (4.7) | [M − H − CH3 − CO]−˙ | ||||||
| 348 | 151 (1.0) | RDA | ||||||
| ISOFLAVONES | ||||||||
| Genistein (2) |
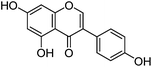
|
14.03 | 0.5 (1.6) | 14.16 | 269 | 269 (100) | [M − H]− | |
| 241 (2.0) | [M − H − CO]− | |||||||
| 224 (6.4) | [M − 2H − CO2]− | |||||||
| 260 | 213 (3.9) | [M − H − 2CO]− | ||||||
| 332 (sh) | 201 (8.2) | [M − H − C3O2]− | ||||||
| 197 (6.8) | [M − H − CO − CO2]− | |||||||
| 159 (13.8) | [M − H − C3O2 − C2H2O]− | |||||||
| 133 (20.4) | iFF | |||||||
| 107 (9.1) | RDA − CO2 | |||||||
| Isoprunetin (6) |
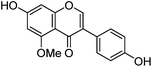
|
12.17 | — | 12.16 | 283 | 283 (30.7) | [M − H]− | |
| 255 | 268 (63.0) | [M − H − CH3]−˙ | ||||||
| 332 (sh) | 240 (100) | [M − H − CH3 − CO]−˙ | ||||||
| 196 (6.7) | [M − H − CH3 − CO − CO2]−˙ | |||||||
| Glycitein (7) |
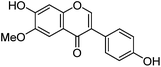
|
12.91 | — | 235 (sh) | 12.98 | 283 | 283 (2.6) | [M − H]− |
| 257 | 268 (100) | [M − H − CH3]−˙ | ||||||
| 320 | 240 (58.2) | [M − H − CH3 − CO]−˙ | ||||||
| 196 (4.3) | [M − H − CH3 − CO − CO2]−˙ | |||||||
| Prunetin (8) |
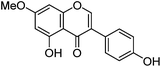
|
18.93 | — | 19.04 | 283 | 283 (6.4) | [M − H]− | |
| 260 | 268 (100) | [M − H − CH3]−˙ | ||||||
| 332 (sh) | 240 (7.7) | [M − H − CH3 − CO]−˙ | ||||||
| 196 (1.2) | [M − H − CH3 − CO − CO2]−˙ | |||||||
| Biochanin A (9) |
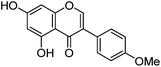
|
19.30 | — | 19.45 | 283 | 283 (9.5) | [M − H]− | |
| 260 | 268 (100) | [M − H − CH3]−˙ | ||||||
| 332 (sh) | 240 (5.3) | [M − H − CH3 − CO]−˙ | ||||||
| 239 (7.3) | [M − 2H − CH3 − CO]− | |||||||
| 211 (3.5) | [M − 2H − CH3 − 2CO]− | |||||||
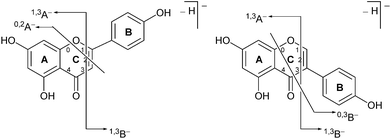 | ||
| Fig. 2 Formal notation used for the retrocyclization fragmentation of the [M − H]− ions of (a) flavonoids and (b) isoflavonoids under negative ion electrospray ionisation conditions. | ||
Alongside the standards used for optimisation of the UPLC conditions, several O-methylated isoflavones related to genistein (2) [isoprunetin (6), glycitein (7), prunetin (8) and biochanin A (9)], which were putative candidates for the minor components found in historical extracts, were selected for study by UPLC-MS. With this larger group of compounds some general trends in elution times could be deduced. Not surprisingly, compounds with an increased hydroxyl substitution pattern were shown to elute at shorter retention times under the UPLC conditions employed (cf. luteolin vs. apigenin, Table 1). Methylation generally gave rise to longer retention times (cf. genistein vs. prunetin, Table 1), except for where methylation disrupted the chelating motif formed by the 5-OH and the carbonyl of the C-ring (cf. genistein vs. isoprunetin, Table 1), in these cases shorter retention times were observed due to reduced binding to the stationary phase.
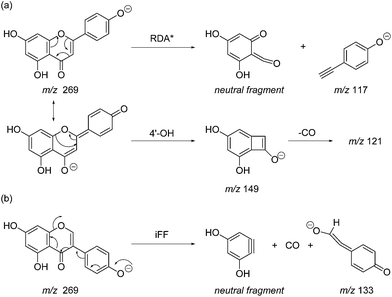
Prior to sample analysis, ESI conditions were optimised using a reference standard solution containing luteolin, genistein, apigenin, chrysoeriol and diosmetin at 10 μg mL−1 amongst others to maximise signal and minimise in-source fragmentation. Standard solutions were then used to characterise fragmentation patterns in the MS/MS experiments. For the MS/MS studies the deprotonated standards, [M − H]−, were subjected to fragmentation at collision energies ranging from 10–40 eV; with 25 eV determined to be optimal as it resulted in significant fragmentation across all species. The results of MS/MS fragmentation at this collision energy are presented in Table 1, with fragment ion intensities reported as a percentage of the base peak intensity (% BPI). Direct comparison of MS/MS fragmentation of deprotonated flavonoids and isoflavonoids under these conditions has allowed us to identify the following trends. When deprotonated on the A-ring, both flavonoids and isoflavonoids show cleavage due to a retro-Diels–Alder mechanism (RDA, 1,3A−, Scheme 1); this is more pronounced in the case of flavonoids and is frequently accompanied by the loss of CO2.20 However, when deprotonated on the B-ring, flavonoids show breakdown by the equivalent retro-Diels–Alder mechanism (RDA*, 1,3B−, Scheme 2), whereas there is little evidence for this in the isoflavonoids. Instead, an alternative 4′-OH isoflavonoid-specific fragmentation pathway predominates (iFF, 0,3B−, Scheme 2). This is in direct contrast to our previously reported 4′-OH flavonoid-specific pathway (4′-OH, 0,2A−, Scheme 2).20 As expected, upon methylation both flavonoids and isoflavonoids have as a principal fragment the [M − H − CH3]−˙ ion, which typically occurs as the base peak and is accompanied by neutral losses of CO and CO2; retrocyclization accounts for only a very small proportion of the fragmentation in these cases.
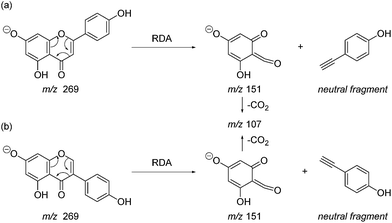 | ||
| Scheme 1 Retro-Diels–Alder (RDA, 1,3A−) MS/MS fragmentation mechanisms for [M − H]− with ionisation at the 7-OH, illustrated for: (a) the flavonoid apigenin; and (b) the isoflavonoid genistein. | ||
3.2 Dye components characterised in Genista tinctoria L.
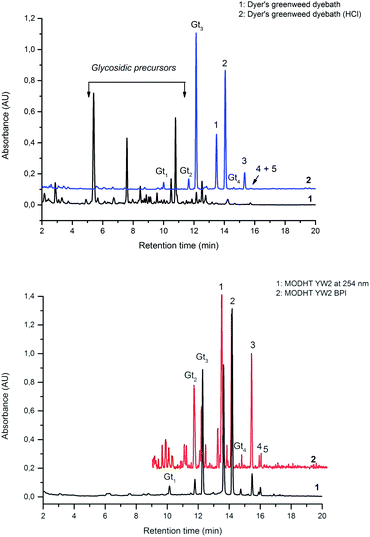
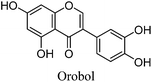
Fragmentation of Gt2. The minor component Gt2 exhibits a maximum absorption at 260 nm which indicates an isoflavonoid structure, with [M − H]− at m/z 285 that corresponds to the isoflavone genistein with an additional OH. Several isomeric isoflavone structures corresponding to this level of hydroxyl substitution have been reported in the literature of which 5,7,3′,4′-tetrahydroxyisoflavone (orobol); 6,7,3′,4′-tetrahydroxyisoflavone; 2,5,7,4′-tetrahydroxy-isoflavone and 7,8,3′,4′-tetrahydroxyisoflavone exhibit a maximum absorption between 258 and 262 nm.26 Detailed MS/MS studies showed a fragmentation pattern similar to genistein (Table 2); and the decrease in retention time under UPLC-MS conditions of 2.2 min relative to genistein compares favourably with the flavonoid pairing of luteolin and apigenin where an additional hydroxyl group in the B-ring decreases the retention time by 2.02 min (Table 1). These data, combined with the UV-vis spectra, suggest that Gt2 corresponds to 5,7,3′,4′-tetrahydroxyisoflavone (orobol), a hydroxylated isoflavone that has been identified in several Genista species growing in Italy and also in Genista tinctoria.27,28
| Unknown component | PDA Rt (min) | λ max MeOH (nm) | MS/MS Rt (min) | [M − H]−m/z | MS/MS (CE: 25 eV) m/z (% BPI) fragment | Proposed structure | |
|---|---|---|---|---|---|---|---|
| a The ions annotated with iFF correspond to the isoflavonoid-specific fragmentation (0,3B−). | |||||||
| Gt1 | 10.06 | 218 (sh) | — | — | — | Not identified | |
| 255 | |||||||
| 290 (sh) | |||||||
| Gt2 | 11.67 | 11.96 | 285 | 285 (100) | [M − H]− |
|
|
| 268 (8.5) | |||||||
| 260 | 257 (13.2) | [M − H − CO]− | |||||
| 290 (sh) | 240 (6.4) | [M − 2H − CO2]− | |||||
| 330 (sh) | 229 (12.3) | [M − H − 2CO]− | |||||
| 217 (12.7) | [M − H − C3O2]− | ||||||
| 198 (9.8) | |||||||
| 149 (14.3) | iFF | ||||||
| Gt3 | 12.17 | 12.38 | 283 | 283 (28.5) | [M − H]− |
|
|
| 268 (64.8) | [M − H − CH3]−˙ | ||||||
| 255 | 240 (100) | [M − H − CH3 − CO]−˙ | |||||
| 330 (sh) | 211 (3.4) | [M − 2H − CH3 − 2CO]− | |||||
| 196 (14.8) | [M − H − CH3 − CO − CO2]−˙ | ||||||
| 184 (10.1) | |||||||
| Gt4 | 14.61 | 14.89 | 299 | 299 (12.8) | [M − H]− |
|
|
| 219 (sh) | 284 (100) | [M − H − CH3]−˙ | |||||
| 261 | 256 (7.3) | [M − H − CH3 − CO]−˙ | |||||
| 295 (sh) | 255 (6.0) | [M − 2H − CH3 − CO]− | |||||
| 340 (sh) | 227 (5.5) | [M − 2H − CH3 −2CO]− | |||||
| 200 (6.4) | |||||||
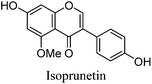
Fragmentation of Gt3. The minor component Gt3 exhibits a maximum absorption at 254 nm, with [M − H]− at m/z of 283 that corresponds to an O- or C-methylated isoflavonoid related to genistein. Detailed MS/MS studies (Fig. 4) showed the presence of a [M − H − CH3]−˙ fragment ion at m/z 268, characteristic of an O-methylated isoflavone. In addition, the base peak at CE 25 eV was found to be at m/z 240 corresponding to the fragment ion [M − H − CH3 − CO]−˙. The close match of the MS2 fragmentation pattern to that of the authentic standard, combined with the correlation of retention time and the UV-vis data allowed Gt3 to be unambiguously identified as 5-O-methyl genistein or isoprunetin (6). This was confirmed by a spiking experiment under the UPLC conditions in which authentic isoprunetin was added to the extract from reference yarn YW2 (ESI 4†). Although it has not previously been identified in historical textile extracts, the presence of high levels of isoprunetin and its associated glycoside in the Genista tinctoria L. textile extract is not surprising, as it is one of the main components reported in Genistea species,29 and has been previously characterised in several Genista species and also Genista tinctoria L. plant extracts.28,30
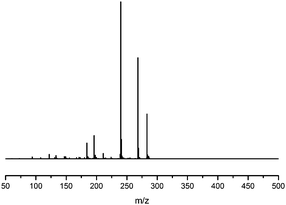 | ||
| Fig. 4 MS/MS fragmentation of the [M − H]− ion (m/z 283) of unknown component Gt3 under negative ion electrospray ionisation conditions (CE: 25 eV). | ||
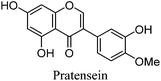
Fragmentation of Gt4. Finally, the minor component Gt4 eluted closely to genistein (2) in the UPLC chromatogram at 14.61 min and exhibited a maximum absorption at 261 nm and [M − H]− at m/z 299. Further MS/MS study showed a fragmentation pattern similar to biochanin A (8) with an extra hydroxyl group; the base peak was found to be a [M − H − CH3]−˙ fragment ion at m/z 284, characteristic of an O-methylated isoflavone. This could correspond to pratensein (3′-hydroxybiochanin A), which is the isoflavonoid equivalent of diosmetin (5).31 Again a significant decrease in retention time under UPLC-MS conditions relative to biochanin A would support additional hydroxylation in the B-ring; in addition, an increased retention time of 2.93 min relative to orobol (Table 2) fits well with the equivalent flavonoid pairing of luteolin and diosmetin where methylation of the 4′-OH leads to an increased retention time of 2.37 min (Table 1). However, alternative structures for Gt4 include 3′-O-methylorobol, the isoflavonoid equivalent of chrysoeriol (4), or 7-O-methylorobol possibly formed by alkylation of a 7-O-glycosidic species32 under the acidic extraction conditions, as recently reported in a study of the constituents of sawwort (Serratula tinctoria L.).33 However, it is clear that it is not the 5-O-methylated derivative as this, by analogy with isoprunetin, would be expected to elute much earlier in the UPLC chromatogram.
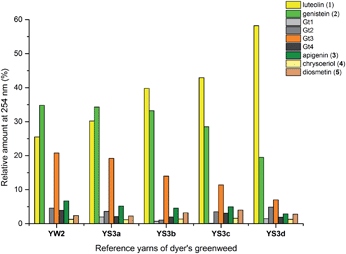
3.3 Effect of textile preparation and ageing on the dye fingerprint
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) genistein. However, in light of the photo-degradation studies presented above, the scattering of the composition might also reflect variation in the levels of photo-degradation of the samples.
genistein. However, in light of the photo-degradation studies presented above, the scattering of the composition might also reflect variation in the levels of photo-degradation of the samples.
4. Conclusions
This UPLC study provides a greatly improved method for the identification of a range of flavonoid and isoflavonoid dyestuffs from historical textile samples, including the regio-isomeric compounds chrysoeriol and diosmetin. This method has allowed the unambiguous identification of additional dye components which occur in the plant extract of Genista tinctoria L. and also in the acid hydrolysed extracts of reference and historical yarns dyed with this species. Four unknowns were also identified in the extracts, designated Gt1–4 on the basis of their relative retention times, and further study of these by UPLC-MS/MS allows us to suggest a structural identification of Gt2 as orobol, Gt3 as isoprunetin and Gt4 as pratensein (or a related O-methylated isoflavone). We believe that these, in particular Gt3, might aid with the identification of dyer's greenweed as a plant dye source on historical textiles. This robust method was also used to study the effects of over-dyeing and photo-degradation on the relative compositions of dyestuff components on wool and silk substrates, allowing us to contextualise a selection of historical yarns sampled from mid-sixteenth century English tapestries. The application of UPLC and MS techniques to the study of historical textile samples opens up exciting possibilities for heritage applications, as objects from which sampling was previously not possible may now be examined due to the greatly reduced requirements for sample size and subsequent limit of detection obtained, enabling the identification of very minor components in heavily degraded samples. Given the clear benefits which this study demonstrates and the wealth of information which these methods provide, we anticipate that those working in the field of heritage science will rapidly adopt UPLC as the method of choice for natural product dyestuff analysis.Acknowledgements
We thank Jane Rowlands and Patricia Collins (Burrell Collection, Glasgow, UK) and David Howell (Bodleian Library, Oxford, UK) for allowing sampling of the tapestries; Andrew Simpson from Waters UK for technical support on both HPLC and UPLC systems; Lorraine Gibson, Strathclyde University, Glasgow UK, for advice during method development; Logan Mackay, University of Edinburgh for assistance with UPLC-MS. Financial assistance was provided through the Science and Heritage programme (studentship to LGT, Grant ref. CDA08/411), the EC (contract number EVK4-CT-2001-00048, Monitoring of Damage to Historic Tapestries project), Glasgow Museums and National Museums Scotland. Tapestry detail in the graphical abstract reproduced courtesy of Glasgow Museums Collection.Notes and references
- N. C. Veitch, Nat. Prod. Rep., 2013, 30, 988–1027 RSC.
- V. Sharma and K. G. Ramawat, in Natural Products, ed. K. G. Ramawat and J. M. Mérillon, Springer-Verlag Berlin Heidelberg, 2013, pp. 1849–1865 Search PubMed.
- E. S. B. Ferreira, PhD thesis, University of Edinburgh, 2002.
- D. A. Peggie, PhD thesis, University of Edinburgh, 2006.
- D. Cardon, Dyes Hist. Archaeol., 1995, 13, 59–73 Search PubMed.
- E. S. B. Ferreira, H. McNab, A. N. Hulme and A. Quye, Chem. Soc. Rev., 2004, 33, 329–336 RSC.
- D. Cardon, Le monde des teintures naturelles, Belin Paris, 2003 Search PubMed.
- J. H. Hofenk de Graaf, The colourful past, Archetype Publications, 2005 Search PubMed.
- A.-M. Hacke and A. Quye, Appendix 2 in Wroughte in Gold and Silk: Preserving the Art of Historic Tapestries, ed. A. Quye, K. Hallet and C. Herrero Carretero, NMS Enterprises Ltd. – Publishing, Edinburgh, UK , 2009 Search PubMed.
- N. Al-Maharik and N. P. Botting, Tetrahedron, 2003, 59, 4177–4181 CrossRef CAS.
- B. J. Compton, L. Larsen and R. T. Weavers, Tetrahedron, 2011, 67, 718–726 CrossRef CAS PubMed.
- S. A. Adensanya, M. J. O'Neill and M. F. Roberts, Phytochemistry, 1985, 24, 2699–2702 CrossRef.
- J. Wouters and A. Verhecken, Stud. Conserv., 1989, 34, 189–200 CrossRef CAS PubMed.
- D. A. Peggie, A. N. Hulme, H. McNab and A. Quye, Microchim. Acta, 2008, 162, 371–380 CrossRef CAS.
- C. Hartmann, J. Smeyers-Verbeke, D. L. Massart and R. D. McDowall, J. Pharm. Biomed. Anal., 1998, 17, 193–218 CrossRef CAS.
- A. Serrano, M. van Bommel and J. Hallett, J. Chromatogr. A, 2013, 29, 102–111 CrossRef PubMed.
- L. G. Troalen, PhD thesis, The University of Edinburgh, 2013.
- A. Villela, E. J. C. van der Klift, E. S. G. M. Mattheussens, G. C. H. Derksen, H. Zuilhof and T. A. van Beek, J. Chromatogr. A, 2011, 1218, 8544–8550 CrossRef CAS PubMed.
- A. N. Hulme, H. McNab, D. A. Peggie and A. Quye, Phytochemistry, 2005, 66, 2766–2770 CrossRef CAS PubMed.
- H. McNab, E. S. B. Ferreira, A. N. Hulme and A. Quye, Int. J. Mass Spectrom., 2009, 284, 57–65 CAS.
- K. Ablajan, J. Mass Spectrom., 2011, 46, 77–84 CrossRef CAS PubMed.
- R. E. March, M. Xiu-Sheng, C. D. Metcalfe, M. Stobiecki and L. Marczak, Int. J. Mass Spectrom., 2004, 232, 171–183 CAS.
- J. Kang, L. A. Hick and W. E. Price, Rapid Commun. Mass Spectrom., 2007, 21, 857–868 CrossRef CAS PubMed.
- M. Łuczkiewicz, D. Głód, T. Bączek and A. Buciński, Chromatographia, 2004, 60, 179–185 Search PubMed.
- F. Tosum, C. Akyuz Kizilay and A. U. Tosun, Chem. Nat. Compd., 2009, 45, 83–84 CrossRef.
- S. E. Kulling, D. M. Honig, T. J. Simat and M. Metzler, J. Agric. Food Chem., 2000, 48, 4963–4972 CrossRef CAS PubMed.
- C. Noccioli, L. Luciardi, S. Barsellini, C. Favro, A. Bertoli, A. Bader, M. C. Loi and L. Pistelli, Chem. Nat. Compd., 2012, 48, 672–673 CrossRef CAS.
- D. Rigano, V. Cardile, C. Formisano, M. T. Maldini, S. Piacente, J. Bevilacqua, A. Russo and F. Senatore, Chem.-Biol. Interact., 2009, 180, 211–219 CrossRef CAS PubMed.
- J. B. Harborne, Phytochemistry, 1969, 8, 1449–1456 CrossRef CAS.
- O. Boumaza, R. Mekkiou, R. Seghiri, D. Sarri, S. Benayache, V. P. Garcia, J. Bermejo and F. Benayache, Chem. Nat. Compd., 2006, 42, 730–731 CrossRef CAS PubMed.
- M. Chen, L. Liu and X. Chen, Can. J. Chem., 2013, 11, 1147–1154 CrossRef.
- I. I. Ozimina and V. A. Bandyukova, Khim. Prir. Soedin., 1985, 507–510 CAS.
- K. Lech, K. Witkoś and M. Jarosz, Anal. Bioanal. Chem., 2014, 406, 3703–3708 CrossRef CAS PubMed.
- Y. Ungar, O. F. Osundahunsi and E. Shimoni, J. Agric. Food Chem., 2003, 51, 4394–4399 CrossRef CAS PubMed.
- B. Eisen, Y. Ungar and E. Shimoni, J. Agric. Food Chem., 2003, 51, 2212–2215 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Preparation of an authentic sample of isoprunetin; (ESI−) MS2 fragmentation of flavonoid and isoflavonoid standards at 25 eV; UPLC-PDA and (ESI−) MS2 fragmentation of Gt components at 25 eV; UPLC-PDA investigation of references and historical yarns. See DOI: 10.1039/c4ay01509f |
| ‡ As commonly adopted by the field, the abbreviation UPLC has been used for the technique of ultra-high pressure liquid chromatography (UHPLC). |
| This journal is © The Royal Society of Chemistry 2014 |