Measuring bacterial adaptation dynamics at the single-cell level using a microfluidic chemostat and time-lapse fluorescence microscopy†
Abstract
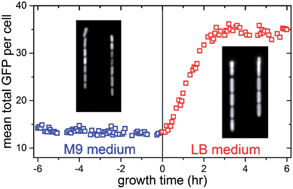
We monitored the dynamics of cell dimensions and reporter GFP expression in individual E. coli cells growing in a microfluidic chemostat using time-lapse fluorescence microscopy. This combination of techniques allows us to study the dynamical responses of single bacterial cells to nutritional shift-down or shift-up for longer times and with more precision over the chemical environment than similar experiments performed on conventional agar pads. We observed two E. coli strains containing different promoter–reporter gene constructs and measured how both their cell dimensions and the GFP expression change after nutritional upshift and downshift. As expected, both strains have similar adaptation dynamics for cell size rearrangement. However, the strain with a ribosomal RNA promoter dependent reporter has a faster GFP production rate than the strain with a constitutive promoter reporter. As a result, the mean GFP concentration in the former strain changes rapidly with the nutritional shift, while that in the latter strain remains relatively stable. These findings characterize the present microfluidic chemostat as a versatile platform for measuring single-cell bacterial dynamics and physiological transitions.

 Please wait while we load your content...
Please wait while we load your content...