Iron catalyzed enantioselective sulfa-Michael addition: a four-step synthesis of the anti-asthma agent Montelukast†
Abstract
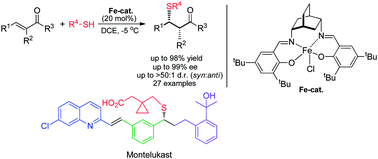
A salen ligand based on a chiral cis-2,5-diaminobicyclo[2.2.2]octane scaffold forms an iron(III) complex with ferric chloride which catalyzes asymmetric addition of thiols to α,β-unsaturated ketones under mild conditions. The reaction (sulfa-Michael addition) produces β-thioketones in excellent yield and high enantiomeric excess from a wide range of aliphatic and aromatic thiols using chalcones and other conjugated enones as Michael acceptors. With α-substituted α,β-unsaturated ketones as acceptors, the addition shows strong preference (typically >50 : 1) for the syn diastereomer over the anti product. An asymmetric synthesis of (R)-Montelukast, the sodium salt of which is the commercial anti-asthma drug Singulair®, was devised using conjugate addition of a thiol catalyzed by our iron(III)–salen complex to an α,β-unsaturated ketone synthesized in a four-component, one-pot tandem Michael-aldol condensation. The reaction sequence to (R)-Montelukast proceeded in 72% overall yield over four steps from commercially available materials. A mechanism for our catalyzed asymmetric sulfa-Michael addition is advanced which coordinates the enone acceptor to the metal centre of the iron–salen complex in an open lower quadrant under the bicyclic scaffold, thereby exposing only the si face of the double bond to attack by the external nucleophilic thiol. Prior internal coordination of the thiol to the metal centre of the complex is proposed based on spectroscopic and chemical evidence and leads to activation of the catalyst through a trans ligand effect.

 Please wait while we load your content...
Please wait while we load your content...