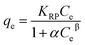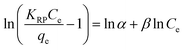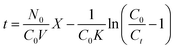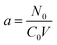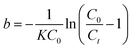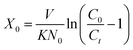DOI:
10.1039/C4RA07395A
(Paper)
RSC Adv., 2014,
4, 49569-49576
A potentially low-cost modified sawdust (MSD) effective for rapid Cr(VI) and As(V) removal from water†
Received
21st July 2014
, Accepted 3rd September 2014
First published on 5th September 2014
Abstract
The study was carried out to evaluate the effectiveness of a diethylenetriamine (DETA)-crosslinked adsorbent prepared from pine sawdust for removing Cr(VI) and As(V) from aqueous solutions. The maximum adsorption capacity of Cr(VI) and As(V) by MSD was highly enhanced from 7.04 mg L−1 to 238.6 mg g−1 and from 4.88 to 71.23 mg g−1 at pH 6.0, respectively. The surface characterization of the MSD proved that the grafted amino groups were responsible for the good affinity towards Cr(VI) and As(V). The uptake of Cr(VI) and As(V) was highly dependent on the pH and was facilitated in acidic solutions. Batch experiments were conducted as a function of pH, temperature and contact time. Both Cr(VI) and As(V) adsorption equilibrium could be quickly attained within 1 h and the process followed the Redlich–Peterson isotherm model. As(V) uptake was more sensitive to an individual coexisting anion (Cl−, SO42−, NO3−, HCO3−) in the system than Cr(VI). The mechanism of adsorption was characterized by the electrostatic attraction between the positively charged surface of sawdust and Cr(VI)/As(V) anions.
1. Introduction
Chromate (Cr(VI)) or arsenate (As(V)) enrichment in aquatic environments is of great concern to people worldwide because of the chronic effects of poisoning on people exposed to high concentrations. Cr(VI) is highly toxic and prolonged exposure to elevated concentrations can cause damage to the liver, kidney and nervous tissue.1,2 The typical symptoms of long-term exposure to arsenic are associated with the onset of various types of diseases (neurological, hematological, renal and skin diseases) and even cancers. In the view of the adverse effects of environmental Cr(VI) and As(V), the World Health Organization (WHO) drinking water guidelines for total chromium and total arsenic are 0.05 mg L−1 and 0.01 mg L−1, respectively.
Concentrations of arsenic above 0.01 mg L−1 may be naturally caused by certain special geological conditions; occurrences of arsenic at elevated concentrations in groundwater are reported in many literatures.3–5 For example, the groundwater from Datong Basin (China) was detected to contain an arsenic concentration up to 1.55 mg L−1.6 However, high levels of Cr(VI) or As(V) discharged into water bodies are more commonly caused by industrial activities, such as leather tanning, mining, electroplating and metal smelting industries.7
Among many technologies available for Cr(VI) or As(V) removal from water, chemical precipitation is a conventional remediation technology, which typically involves the reduction of Cr(VI) to Cr(III) and subsequent precipitation as sludge at high pH. However, this removal method is commonly applied for wastewater with very high Cr(VI) or As(V) concentrations and could not remove them satisfactorily to meet the discharge standards. For instance, the removal of As(V) from water via chemical precipitation induced by adding lime can only reduce solution As(V) concentration to a level of 1–5 mg L−1, still considerably higher than the drinking water standard (0.01 mg L−1). Moreover, the production of large amounts of toxic sludge cannot be ignored.
In recent years, considerable studies have focused on the removal of Cr(VI) or As(V) via adsorption. Various types of adsorbents have been reported including activated carbons, metal oxides, polymeric adsorbents and biocomposites (mycelium, bacteria, agriculture waste, etc.).8–10 In particular, biomass adsorbents have gained considerable attention because of the very low cost and great potential to be modified as a type of highly efficient adsorbent. In this study, sawdust (approximately US$ 0.02–US$ 0.09, China) is selected as a host material because it is produced in large quantities as a solid waste at sawmills. In addition, sawdust mainly contains lignocellulose and the cellulose content of oak (Quercus coccifera) sawdust was reported to be 41.5%.11 A variety of functional groups, such as the hydroxyl group allow the lignocellulose to react with other moieties for the enhancement in the efficiency of metal ions adsorption. Novel adsorbents based on modified sawdust have been reported by several studies. Ansari and Fahim modified sawdust by coating with polypyrrole in order to get an anion exchange material.12 The synthetic route was simple and the modified sawdust was demonstrated to be an effective adsorbent to remove Cr(VI) from aqueous solutions. Bajpai et al. also prepared modified sawdust by polypyrrole coating to remove phosphate from water.13 Khalkhali et al. investigated the removal of Cr(VI) from water using polyaniline coated sawdust.14
Previous studies have demonstrated that the amino group was effective in the removal of metal anions.15–17 In this study, a novel low-cost MSD adsorbent material was developed for the adsorption of Cr(VI) and As(V) from water. The synthetic route includes two steps: etherification and crosslinking reaction. A recent study reported that a crosslinked cornstalk was further grafted with triethylamine to obtain a type of anion exchanger.16 However, our study demonstrated that after crosslinking with DETA sawdust particles aggregated and it was impossible to further react with triethylamine. Additionally, we obtained a good adsorption performance for Cr(VI) almost as high as the triethylamine grafted cornstalk; moreover, the synthetic procedure was simplified. SEM results obviously showed a thin organic film covering the surface of the sawdust, but it did not mean that any other type of support was appropriate because the etherification reaction between the lignocellulose hydroxyl and epichlorohydrin (ECH) was a key step in the occurrence of the following cross-linking reaction.
2. Materials and methods
2.1. Reagents and stock solutions
Stock solutions of 1000 mg L−1 Cr(VI) and 1000 mg L−1 As(V) were prepared using K2Cr2O7 (AR quality), Na2HAsO4 (AR quality) and deionized water. NaOH solutions (0.1 and 0.01 M) and HNO3 solutions (0.1 and 0.01 M) were prepared using sodium hydroxide (AR quality) and nitric acid (AR quality) in order to adjust the pH values of aqueous media. Cr(VI) samples of various concentrations were prepared with deionized water. Organic reagents (ECH, DMF, DETA, etc.) used in the synthesis process were all of analytical reagent grades.
2.2. Preparation of crosslinked sawdust
The sawdust was sized through a 40-mesh screen and washed with deionized water to remove impurities. The pretreated sawdust (3 g) was dispersed in a mixture solutions of 20 mL N,N-dimethylformamide (DMF) and 15 mL ECH in a three-necked flask was placed in a constant-temperature water-bath at 90 °C for 1 h. The crosslinking agent, 5 mL diethylenetriamine (DETA), was added dropwise into the flask and placed for 1 h. Finally, the mixture was filtered and the precipitated product was washed with absolute ethyl alcohol, and then dried. The synthesis route is shown in Fig. 1.
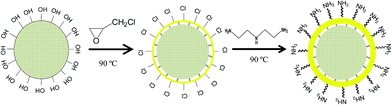 |
| | Fig. 1 Schematic diagram of the synthesis process of MSD. | |
2.3. Batch experiments
2.3.1. Isotherm study. Batch experiments were carried out by mixing 100 mL K2Cr2O7 or Na2HAsO4 solution of various concentrations with 0.2 g MSD at pH 6.0 and room temperature. The isotherm experiments were conducted with initial Cr(VI) and As(V) concentrations from 1 to 200 mg L−1.
2.3.2. Kinetics. The effect of the contact time on the removal of Cr(VI) and As(V) was investigated with 10, 50 and 100 mg L−1 of Cr(VI) and As(V) solutions. The experiments were conducted in a round glass reactor that was placed in a thermostatic water bath where the temperature could be adjusted to specific temperatures. A pH electrode combined with a thermometer was inserted into the reactor solution to detect the pH and temperature change. Samples were collected at certain time intervals.
2.3.3. Effect of pH and coexisting anions. The solution pH (100 mg L−1 Cr(VI) and 50 mg L−1 As(V)) was adjusted with the addition of NaOH or HNO3 solution to obtain the pH range of 2–11. The sample's pH was adjusted several times during the initial 2 h to maintain the desired pH. Coexisting anion solutions were prepared including the following anions Cl−, SO42−, NO3− and HCO3−.
2.3.4. Fixed-bed column run. The packed column experiments were conducted using a glass column of 22 × 200 mm, packed with a certain dosage of MSD to obtain different bed depths. A nylon mesh (200 μm opening size) was placed at the bottom of the glass column to prevent sawdust from being discharged into the tubing. A Cr(VI) (10 mg L−1) or As(V) (2.5 mg L−1) solution at natural pH was pumped through the column at a desired flow rate (10.1 mL min−1) with a peristaltic pump. Effluent samples were collected at regular time intervals to determine the Cr(VI) or As(V) concentration in the effluent solutions.
2.4. Analysis methods
FTIR spectra were obtained by a UV2550 instrument (Shimadzu Co., Ltd., Japan) using KBr discs as background over the range of 450–4000 cm−1. The mass ratio between the sample and KBr powder was about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. SEM images were obtained using a Quanta 200 FEG scanning electron microscope (USA), which was coupled with an EDS system. The BET surface areas were determined by an ASAP 2020 Accelerated Surface Area and Porosimetry System instrument (Global Spec. Inc., US). All the samples were analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Optima 2000, UK).
100. SEM images were obtained using a Quanta 200 FEG scanning electron microscope (USA), which was coupled with an EDS system. The BET surface areas were determined by an ASAP 2020 Accelerated Surface Area and Porosimetry System instrument (Global Spec. Inc., US). All the samples were analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Optima 2000, UK).
3. Results and discussion
3.1. Surface properties of MSD
3.1.1. FTIR analysis. FTIR was carried out to investigate the surface properties of raw sawdust and MSD. As shown in Fig. 2, the broad band at 3310 cm−1 represented the –OH groups. The peak at 2918 cm−1 in the raw sawdust is associated with the stretching vibration of the aliphatic C–H. The peaks at 1738, 1640 and 1503 cm−1 for raw sawdust could be assigned to carboxylic groups. Pine sawdust consists mainly of lignocellulose, hemicelluloses, pectin, lipids and waxes.18 Carboxylic groups are primarily present in pectin, lipids and waxes; the absence of the aforementioned bands (1738, 1640 and 1503 cm−1), which represent carboxylic groups may indicate that these substances have already been removed during the synthetic process. The peaks at 1555 cm−1 and 1542 cm−1 indicate the presence of amino groups in MSD. The intense vibrations observed at 1370 and 1057 cm−1 in MSD could be assigned to the C–N stretching vibration of amino groups. The functional groups before and after modification and their corresponding infrared adsorption bands are shown in Table 1.
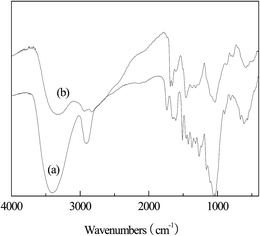 |
| | Fig. 2 FTIR spectra of (a) raw sawdust and (b) MSD. | |
Table 1 FTIR spectral characteristics of sawdust before and after modification
| Wavelength range (cm−1) |
RS |
MSD |
Assignment |
Reference |
| 3500–3300 |
3349 |
3312 |
Stretching of –OH, –NH group |
19 |
| 3000–2880 |
2918 |
2940 |
Aliphatic C–H group |
20 |
| 1750–1680 |
1738 |
Absent |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching O stretching |
21 |
| 1670–1640 |
1640 |
1653 |
Carboxylic groups |
22 |
| 1640–1500 |
1503 |
Absent |
| 1540–1640 |
|
1555 |
N–H bending (in plane) |
This study |
| |
1542 |
| 1470–1400 |
1463 |
1456 |
O–H bending |
21 |
| 1450–1375 |
1426 |
Absent |
Symmetric bending of CH3 |
19 |
| 1375–1300 |
1321 |
Absent |
C–O stretching |
23 |
| 1400–1000 |
1244 |
Absent |
O–H alcohols and aliphatic ether |
23 |
| 1300–1000 |
1162 |
Absent |
C–O stretching of COOH |
23 |
| 1370–1020 |
|
1370 |
C–N stretching |
23 |
| |
1057 |
| 990–560 |
897 |
868 |
C–H bending |
This study |
| 599 |
593 |
3.1.2. Scanning electron microscopy. SEM analysis of the raw sawdust and MSD was performed to clearly see the surfaces of the particles. The SEM images of the raw sawdust revealed that the surface was abundant with regular pores of uniform size. After modification, the surface of sawdust was covered with a thin organic film as shown in Fig. 3. This result validates the increasing trend of the BET surface area from 0.122 to 0.156 m2 g−1 after modification. A similar trend was also observed by Keränen et al.18 who modified the pine sawdust using ECH, ethylenediamine and triethylamine, increasing the BET surface area (P0/P = 0.2) from 0.836 to 1.44 m2 g−1. The summary report of surface areas of the raw sawdust and MSD are shown in Fig. S1.† According to the SEM images, the organic film on the MSD surface appeared to be heterogeneous; thus, Site 1 (S1), Site 2 (S2) and Site 3 (S3) were selected for EDS analysis, as shown in Fig. 3, a slight increase in the C content (54.62%–(56.14–57.78%)) was observed. However, the N content significantly increased from 0.47% to 8.91–13.80%, indicating that a large number of amine groups have been introduced onto the sawdust surface. The N content of MSD varied from 8.91% for S1 to 13.80% for S3, this larger fluctuation could be caused by a side reaction during the synthetic process or the incomplete reaction. The Cl content of MSD slightly fluctuated in a range of 14.63–17.33%, which was because of the excess dosage of ECH reagent. The elemental composition from the EDS analysis is shown in Table 2.
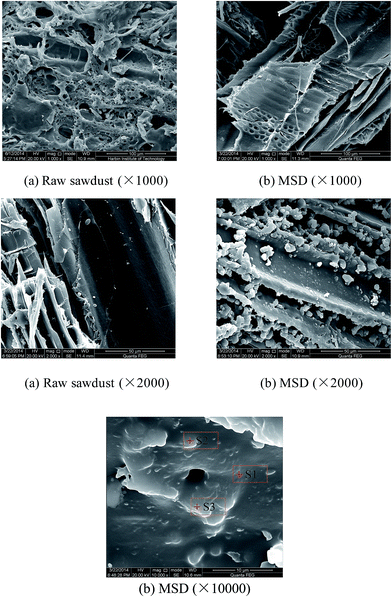 |
| | Fig. 3 SEM images of raw sawdust and MSD. | |
Table 2 Elemental analysis of raw sawdust and MSD (% based on dry weight)
| Element |
Content (wt%) |
| Raw sawdust |
MSD |
| S1 |
S2 |
S3 |
| C |
54.62 |
57.78 |
56.14 |
56.28 |
| O |
42.71 |
18.69 |
14.39 |
12.59 |
| N |
0.47 |
08.91 |
12.46 |
13.80 |
| Cl |
0.2 |
14.63 |
17.01 |
17.33 |
3.2. Batch studies
3.2.1. Effects of solution pH on Cr(VI) and As(V) adsorption. It is important to study the effect of the pH on the removal of Cr(VI)/As(V) onto MSD. The Cr(VI) or As(V) anion is not a simple monovalent anion, but rather a series of anions depending on the pH. Moreover, the pH of the solution affects the surface charge of the adsorbent. Cr(VI) or As(V) speciation is affected by the solution pH through the following equilibrium:| | |
HCrO4− ↔ CrO42− + H+ pKa = 5.9
| (1) |
| | |
H2CrO4− ↔ HCrO42− + H+ pKa = 4.1
| (2) |
| | |
Cr2O72− + H2O ↔ 2HCrO4− pKa = 2.2
| (3) |
| | |
H3AsO4 ↔ H2AsO4− + H+ pKa = 2.3
| (4) |
| | |
H2AsO4– ↔ HAsO42− + H+ pKa = 6.8
| (5) |
| | |
HAsO42− ↔ AsO43− + H+ pKa = 11.6
| (6) |
The influence of pH on the adsorption is shown in Fig. 4, Cr(VI) removal efficiency was constant in neutral and acidic conditions (pH range from 2–6), but significantly decreased from pH 7 to 12. The maximum Cr(VI) adsorption capacity (97.91 mg g−1) was observed at lower pH values, between 2 and 6. A different trend was observed for As(V) adsorption, the optimal pH was found between 4 and 6. When the pH was lowered from 4 to 2, a gradual decrease in the adsorption capacity is observed from 91.8% to 79.2%. According to eqn (4), As(V) species at pH 2–3 exist mostly as the neutral form (H3AsO4). From eqn (5) to (6), As(V) species mainly occur in the form of H2AsO4− in the pH range between 3 and 6, while a divalent anion HAsO42− dominates at higher pH values (pH 7–11). The high concentration of H+ causes the protonation of the MSD surface, which strengthens the electrostatic attraction forces between amino groups and As(V) species. As the pH increases from 3 to 6, the increase in concentration of H2AsO4− and H2AsO4− enhances the electrostatic attraction between the anion exchanger and arsenate. When the pH increases from 7 to 10, the increase of OH− concentration will significantly decrease the protonation effect of amino groups and the excess OH− ions will compete with arsenate for sorption sites.
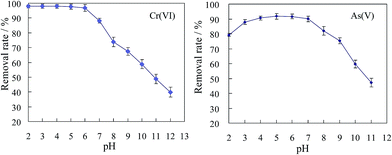 |
| | Fig. 4 Effect of pH on Cr(VI) and As(V) removal by MSD. (Initial Cr(VI) and As(V) concentrations were 100 mg L−1 and 50 mg L−1, respectively, MSD dosage 0.2 g, temperature 25 ± 2 °C.) | |
3.2.2. Adsorption kinetics. In order to define the adsorption rate and the contact time required to reach equilibrium, the kinetic parameters were studied by monitoring the adsorption capacities of Cr(VI) and As(V) in certain time interval. The Cr(VI) adsorption kinetic experiments under temperatures of 10, 30, 50 °C with an initial Cr(VI) concentration of 50 mg L−1 are illustrated in Fig. 5. The slope of the three curves gradually became steep, indicating that the rate of reaction increased with a rise in temperature. The Cr(VI) concentration in solution quickly reached its lowest level (0.65 mg L−1) within 30 min. The rate of reaction of As(V) is also very high and the equilibrium is reached within 40 min. It can be observed that the rate of Cr(VI) adsorption on MSD was higher than that of As(V), the adsorption rate constants at 30 °C for Cr(VI) and As(V) with the same initial concentration of 50 mg L−1 are 9.7 mg g−1 min−1 and 3.8 mg g−1 min−1, respectively. The adsorption rate constant v0 can be obtained from the following equation:
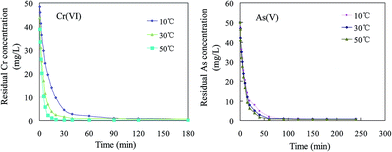 |
| | Fig. 5 Adsorption kinetics of Cr(VI) and As(V) by MSD at different temperatures. (Initial Cr(VI) and As(V) concentrations were 50 mg L−1, MSD dosage 0.2 g, pH 6.0 ± 0.2.) | |
For other batch experiments, the contact time was maintained as 1 h.
The data were regressed against pseudo-first-order, pseudo-second-order and intraparticle diffusion equations (eqn (8)–(10)).
| |
log(qe − qt) = (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe) − k1t qe) − k1t
| (8) |
| |
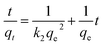 | (9) |
where
qt is the quantity of Cr(
VI) or As(
V) adsorbed at time
t (mg g
−1),
qe is the quantity of Cr(
VI) or As(
V) adsorbed at equilibrium (mg g
−1),
k1 (min
−1),
k2 (g mg
−1 min
−1) and
kd (mg L
−1 min
−1/2) are the rate constants of the pseudo-first-order, pseudo-second-order and intraparticle diffusion equations, respectively.
There are three main stages in the process of adsorption by adsorbents: (1) mass transfer across the external boundary layer; (2) diffusion within the pores of the adsorbent; (3) adsorption at a special site on the surface.19 The external mass-transfer constant (ks) involved in stage (1) is calculated from the initial slope of the curve Ct/C0 versus t according to the following equation:
| |
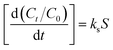 | (11) |
Higher values of ks indicate a decrease in the resistance of the boundary film to mass transfer. The agitation condition of the liquid–solid system caused a decrease in the thickness of the diffusion layer at the adsorbent surface with a consequent increase in the mass-transfer coefficient.20 The intraparticle diffusion rate constant (kd) was calculated from eqn (10). For any porous material, the intraparticle diffusion involved in stage (2) usually plays an important role than surface diffusion, a larger kd and a better conformation to the intraparticle diffusion equation represents that the intraparticle diffusion is the rate-limiting process for adsorption.
As shown in Table 2, the entire kinetic data were best fitted to the pseudo-second-order model, indicating that the adsorption process followed second-order chemisorptions.21 Correlation coefficients R2 lower than 0.73 indicated the bad fit to the intraparticle diffusion equation because MSD had a very small percentage of micropores, mesopores and transitional pores, the surface areas of 0.156 m2 g−1 was mainly attributed to the external surface area.
3.2.3. Adsorption isotherms. Adsorption isotherms at three different temperatures (10, 30 and 50 °C) were obtained to study the thermodynamic process. As shown in Fig. 6, the maximum Cr(VI) adsorption capacity of MSD at 50 °C reached 238.6 mg g−1, while As(V) achieved a maximum adsorption capacity of 71.23 mg g−1. Temperature played an important role in determining the maximum adsorption capacity. As shown in Table 3, the isotherm data fitted the Langmuir model well (R2 > 0.97). Both the KL and qe values increased as the temperature increased, indicating the endothermic nature of Cr(VI)/As(V) adsorption on MSD.
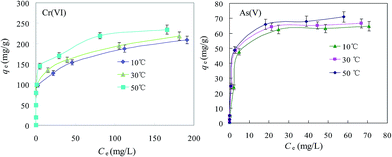 |
| | Fig. 6 Adsorption isotherms for Cr(VI) and As(V) at different temperatures. (MSD dosage 0.5 g, pH 6.0 ± 0.2.) | |
Table 3 Kinetics model fitting parameters for Cr(VI)/As(V) adsorption on MSD at different temperatures
| Species |
T/K |
Pseudo first-order |
Pseudo second-order |
Intraparticle diffusion |
The external mass-transfer equation |
| k1 |
R2 |
k2 |
v0 |
R2 |
kd |
R2 |
ks |
R2 |
| Cr(VI) |
283 |
0.062 |
0.993 |
0.012 |
7.87 |
0.999 |
1.41 |
0.610 |
0.073 |
0.929 |
| 303 |
0.070 |
0.982 |
0.015 |
9.71 |
0.999 |
1.34 |
0.584 |
0.074 |
0.922 |
| 323 |
0.073 |
0.989 |
0.016 |
10.64 |
0.999 |
1.32 |
0.573 |
0.075 |
0.945 |
| As(V) |
283 |
0.018 |
0.970 |
0.0058 |
3.82 |
0.999 |
1.589 |
0.731 |
0.047 |
0.939 |
| 303 |
0.031 |
0.970 |
0.0071 |
4.69 |
0.999 |
1.548 |
0.690 |
0.050 |
0.937 |
| 323 |
0.06 |
0.993 |
0.0089 |
5.88 |
0.999 |
1.489 |
0.654 |
0.052 |
0.923 |
The adsorption equilibrium data were fitted to Langmuir, Freundlich and Redlich–Peterson models. The Langmuir isotherm is derived from the assumption that monolayer adsorption takes place on a homogeneous adsorbent surface with identical adsorption sites. The Langmuir isotherm equation is expressed as follows:21
| |
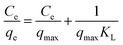 | (12) |
where
qe is the quantity of the species adsorbed at equilibrium (mg g
−1),
KL is a constant representing the virtual bonding strength between the target species and adsorbent,
Ce is the equilibrium concentration of adsorbate in the solution,
qmax is the maximum adsorption capacity.
The assumption of Freundlich isotherm is that the adsorbent surface is distributed with heterogeneous adsorptive energies. The Freundlich isotherm equation was expressed as follows:
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n KF + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (13) |
where
qe is the quantity of the species adsorbed at equilibrium (mg g
−1),
KF is a constant, which is a measure of the adsorption capacity, 1/
n is a measure of adsorption density,
Ce is the equilibrium concentration of adsorbate in the solution.
Redlich–Peterson model incorporates the advantages of both Langmuir and Freundlich models approaching the Freundlich model at high concentrations and agrees well with the Langmuir model at low concentrations.22 Redlich–Peterson equation can be written as follows:
| |
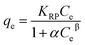 | (14) |
where
KRP,
α is Redlich–Peterson equation constant,
β is the exponent which lies between 0 and 1. The linear form of R–P isotherm equation is as follows:
| |
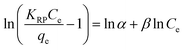 | (15) |
The adsorption of Cr(VI) or As(V) fitted very well with the Langmuir isotherm model with a coefficient of determination (R2) for each set of the linearized data above 0.96 as shown in Table 4.
Table 4 Freundlich, Langmuir and Redlich–Peterson isotherm constants of Cr(VI)/As(V) adsorption on MSD at different temperatures
| Species |
Langmuir isotherm |
Freundlich isotherm |
Redlich–Peterson isotherm |
| T/K |
Qmax (mg g−1) |
kL (L mg−1) |
R2 |
n |
KF (mg g−1) |
R2 |
KRP |
α |
β |
R2 |
| Cr(VI) |
283 |
192.3 |
0.173 |
0.992 |
5.682 |
78.26 |
0.935 |
71 |
0.29 |
1.093 |
0.989 |
| 303 |
232.6 |
0.187 |
0.985 |
5.464 |
86.49 |
0.958 |
225 |
1.94 |
0.906 |
0.999 |
| 323 |
250 |
0.2 |
0.985 |
5.405 |
93.69 |
0.970 |
350 |
2.56 |
0.954 |
0.996 |
| As(V) |
283 |
68.97 |
0.279 |
0.994 |
1.661 |
8.662 |
0.875 |
33 |
0.78 |
0.886 |
0.993 |
| 303 |
71.43 |
0.269 |
0.997 |
1.842 |
11.79 |
0.842 |
89 |
2.09 |
0.878 |
0.996 |
| 323 |
76.92 |
0.25 |
0.997 |
1.876 |
13.80 |
0.827 |
105 |
2.02 |
0.915 |
0.999 |
Fig. S2† shows that the raw sawdust had little affinity for Cr(VI) and As(V). In contrast, the MSD showed an excellent performance for the Cr(VI) or As(V) adsorption. The noteworthy difference indicated that amino groups have good affinity for Cr(VI) and As(V). Because the MSD has a considerably lower BET specific surface area (0.156 m2 g−1) than the strong base anion exchange resin (D211) (66.7 m2 g−1), the high adsorption capacity was attributed to the high density of crosslinking amino groups.
3.2.4. Effects of coexisting anions on the Cr(VI) and As(V) adsorption. In natural water sources such as rivers and lakes, many anions might coexist. In our study, the adsorption of Cr(VI) and As(V) on MSD has been studied in the presence of various inorganic anions including SO42−, NO3−, Cl− and HCO3−. Fig. 7 shows the effects of these competing ions on the removal of Cr(VI) and As(V). The presence of NO3− or Cl−, even at high concentrations, had almost no negative effect on the adsorption capacity of Cr(VI). SO42− at 100 mg L−1 slightly affected the removal of Cr(VI). HCO3− had a stronger effect than the other three anions. For As(V), the presence of SO42−, NO3−, Cl− and HCO3− resulted in a great reduction in As(V) removal efficiency. It was observed that the individual addition of SO42− (10 mg L−1), NO3− (10 mg L−1), Cl− (10 mg L−1) and HCO3− (10 mg L−1) resulted in 18%, 22%, 24% and 28% reduction in the As(V) uptake, respectively, the occurrence of SO42− up to concentration of 100 mg g−1 shows a 74% reduction in the As(V) uptake. All these anions affected the Cr(VI) or As(V) adsorption process via their competition for the electrostatic attraction. According to the results, the following order of magnitude of the interfering effects on MSD was obtained:| | |
Cr2O72− > HCO3− > SO42− > NO3− > Cl− > H2AsO4−
| (16) |
which was slightly different from the order of selectivity on the strong base anion exchange resin:| | |
Cr2O72− > SO42− > NO3− > Cl− > HCO3−
| (17) |
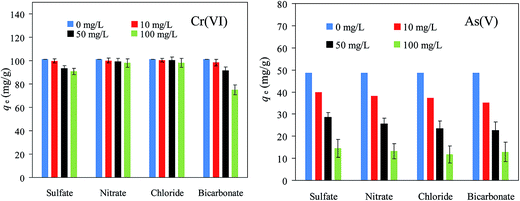 |
| | Fig. 7 Effect of coexisting anions on Cr(VI) and As(V) removal by MSD. (MSD dosage 0.2 g, pH 6.1–7.2, temperature 25 ± 2 °C.) | |
3.3. Fixed bed column runs
Fig. 8 showed the breakthrough curves at different bed depths. The flow rate was maintained at 10.1 mL min−1. It was clear that the bed depth strongly influenced the Cr(VI) adsorption capacity, both the exhaustion time and effluent volume (Veff) increased as the bed depth increased. The slope of the breakthrough curve decreased with an increase in bed depth, which was attributed to the broadened mass transfer zone.23 The adsorption column data were evaluated and are presented in Table 5. Cr(VI) adsorption capacities of 167, 164 and 154 mg g−1 were recorded at bed depths of 1.8, 2.3 and 2.8 cm, respectively. It was shown that the shorter bed depth of 1.8 cm offered an optimum breakthrough curve and the highest adsorption capacity of 167 mg g−1 for Cr(VI). As(V) showed a similar trend of the breakthrough behaviour, the adsorption capacity of As(V) decreased from 75 mg g−1 to 69 mg g−1 with an increase in the bed depth from 1.5 to 2.6 cm.
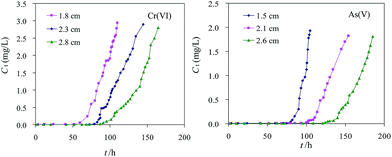 |
| | Fig. 8 Cr(VI) and As(V) breakthrough behavior in the column tests at different bed depths. (Initial Cr(VI) and As(V) concentrations were 10 and 2.5 mg L−1, respectively, pH 6.0 ± 0.2, temperature 25 ± 2 °C.) | |
Table 5 BDST parameters for the sorption of Cr(VI) and As(V) at different bed depths
| Metal |
Bed depth (cm) |
Service time (h) |
Fitted equation |
R2 |
N0 (g L−1) |
Ka (mg−1·h−1) |
X0 (cm) |
| Cr(VI) |
1.8 |
50.1 |
t = 40.54X + 14.09 |
0.998 |
19.56 |
0.037 |
0.35 |
| 2.3 |
63.3 |
| 2.8 |
72.5 |
| As(V) |
1.5 |
75.4 |
t = 22.4X + 10.44 |
0.989 |
10.8 |
0.21 |
0.12 |
| 2.1 |
98.2 |
| 2.6 |
120.1 |
Several models were widely used to simulate column test data, i.e. the Thomas, Yoon–Nelson, Wolborska and Adams–Bohart models. In this study, bed depth service time (BDST) approach based on the Adams–Bohart equation, which was proved to be the most successful method for analyzing the data of column runs,24,25 was used to fit the column experiment data, the model being described by the following equation:
| |
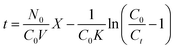 | (18) |
where
C0 is the initial concentration of solute (mg L
−1),
N0 is the saturation concentration of the column bed (mg L
−1),
Ct is the desired concentration of solute at breakthrough (mg L
−1),
Ka is the adsorption rate constant (L mg
−1 h
−1),
X is the column bed depth (cm),
V represents the empty bed flow linear velocity (cm h
−1) and
t the column service time under the abovementioned conditions (h). Service time
t corresponds to the effluent concentrations 0.05 mg L
−1 for Cr(
VI) and 0.01 mg L
−1 for As(
V) according to the drinking water standards.
The BDST model is in the form of:
where
| |
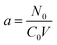 | (20) |
| |
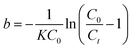 | (21) |
Parameters N0 and Ka can be calculated from the slope of the linear plot of t versus X as shown in Table 5.
Setting t = 0 and solving eqn (19) for X yields:
| |
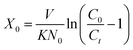 | (22) |
where
X0 is the minimum bed depth allowing an effluent concentration
Ct, known as the critical bed depth. The variation of the service time with bed depth is linearized in Fig. S3.
†
Details on fitted equations between the service time (t) and the bed depth (X) are given in Table 5. The critical depth values obtained for Cr(VI) and As(V) from the BDST model were 0.35 cm and 0.12 cm, respectively.
3.4. Mechanism for chromate and arsenate removal
According to the aforementioned results, we proposed that Cr(VI) and As(V) removal mechanisms might be the electrostatic attraction as shown in eqn (23) and (24). At acidic pH values, the surface charge of MSD was positive because of the protonation effect of amine groups, and then the electrostatic attraction between protonated amine groups and Cr(VI)/As(V) anions contributed to the removal reaction.| | |
–NH3+⋯R− + HCrO4− ↔ –NH3+⋯HCrO4− + R−
| (23) |
| | |
–NH3+⋯R− + H2AsO4− ↔ –NH3+⋯H2AsO4−+ R−
| (24) |
This mechanism also explains that the adsorption process is highly pH dependent and the removal performance significantly decreases in alkaline conditions.
4. Conclusion
The adsorption capacity of MSD for Cr(VI) and As(V) significantly increased when the raw material was crosslinked with DETA. pH of the solution played a key role in the removal of Cr(VI) and As(V) from water, the maximum adsorption being achieved at pH values 2–6 for Cr(VI) and pH 5–6 for As(V). Temperature also plays an important role and the Cr(VI) adsorption capacities increased with a rise in temperature from 10 to 50 °C. The kinetic data can be well described by the pseudo-second-order model indicating the sorption process follows a second-order chemisorption. The adsorption isotherm can be well defined by the Redlich–Peterson model. The MSD exhibits the coexisting anion selectivity sequence bicarbonate > sulfate > nitrate > chloride. The mechanism of the removal of Cr(VI) and As(V) mainly involves the electrostatic attraction between anions and positively charged amine sites.
Acknowledgements
This work was supported by the Funds for Creative Research Groups of China (Grant no. 51121062), National Water Pollution Control and Management Technology Major Projects (2012ZX07205-005). We are very thankful for Emily's language reversion.
References
- M. Owlad, M. K. Aroua, W. A. W. Daud and S. Baroutian, Water, Air, Soil Pollut., 2009, 200, 59–77 CrossRef CAS.
- J. Kotaś and Z. Stasicka, Environ. Pollut., 2000, 107, 263–283 CrossRef.
- N. E. Korte and Q. Fernando, Crit. Rev. Environ. Sci. Technol., 1991, 21, 1–39 CAS.
- D. K. Nordstrom, Science, 2002, 296, 2143–2145 CrossRef CAS PubMed.
- P. Smedley and D. Kinniburgh, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- Y. Deng, Y. Wang and T. Ma, Appl. Geochem., 2009, 24, 587–599 CrossRef CAS PubMed.
- O. V. Lushchak, O. I. Kubrak, O. V. Lozinsky, J. M. Storey, K. B. Storey and V. I. Lushchak, Aquat. Toxicol., 2009, 93, 45–52 CrossRef CAS PubMed.
- X. Dou, R. Li, B. Zhao and W. Liang, J. Hazard. Mater., 2010, 182, 108–114 CrossRef CAS PubMed.
- S. R. Chowdhury and E. K. Yanful, J. Environ. Manage., 2010, 91, 2238–2247 CrossRef CAS PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- M. E. Argun, S. Dursun, C. Ozdemir and M. Karatas, J. Hazard. Mater., 2007, 141, 77–85 CrossRef CAS PubMed.
- R. Ansari and N. K. Fahim, React. Funct. Polym., 2007, 67, 367–374 CrossRef CAS PubMed.
- S. K. Bajpai, V. K. Rohit and M. Namdeo, J. Appl. Polym. Sci., 2009, 111, 3081–3088 CrossRef CAS.
- R. A. Khalkhali, A. R. Aliakbar and M. Masoudi, J. Polym. Mater., 2005, 22, 75–80 CAS.
- X. Xu, B. Gao, W. Wang, Q. Yue, Y. Wang and S. Ni, Colloids Surf., B, 2009, 70, 46–52 CrossRef CAS PubMed.
- S. Chen, Q. Yue, B. Gao, Q. Li and X. Xu, Desalination, 2011, 274, 113–119 CrossRef CAS PubMed.
- A. M. Yusof and N. A. N. N. Malek, J. Hazard. Mater., 2009, 162, 1019–1024 CrossRef CAS PubMed.
- A. Keranen, T. Leiviska, B.-Y. Gao, O. Hormi and J. Tanskanen, Chem. Eng. Sci., 2013, 98, 59–68 CrossRef CAS PubMed.
- E. Malkoc, Y. Nuhoglu and M. Dundar, J. Hazard. Mater., 2006, 138, 142–151 CrossRef CAS PubMed.
- X. Xu, B.-Y. Gao, Q.-Y. Yue, Q.-Q. Zhong and X. Zhan, Carbohydr. Polym., 2010, 82, 1212–1218 CrossRef CAS PubMed.
- V. Vinodhini and N. Das, Am.-Eurasian J. Sci. Res., 2009, 4, 324–329 CAS.
- Z. Akmar Zakaria, M. Suratman, N. Mohammed and W. Azlina Ahmad, Desalination, 2009, 244, 109–121 CrossRef PubMed.
- M. A. Wahab, S. Jellali and N. Jedidi, Bioresour. Technol., 2010, 101, 5070–5075 CrossRef CAS PubMed.
- Q.-S. Liu, T. Zheng, P. Wang, J.-P. Jiang and N. Li, Chem. Eng. J., 2010, 157, 348–356 CrossRef CAS PubMed.
- O. A. Fadali, Adsorpt. Sci. Technol., 2003, 21, 935–950 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07395a |
|
| This journal is © The Royal Society of Chemistry 2014 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. SEM images were obtained using a Quanta 200 FEG scanning electron microscope (USA), which was coupled with an EDS system. The BET surface areas were determined by an ASAP 2020 Accelerated Surface Area and Porosimetry System instrument (Global Spec. Inc., US). All the samples were analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Optima 2000, UK).
100. SEM images were obtained using a Quanta 200 FEG scanning electron microscope (USA), which was coupled with an EDS system. The BET surface areas were determined by an ASAP 2020 Accelerated Surface Area and Porosimetry System instrument (Global Spec. Inc., US). All the samples were analyzed using inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer, Optima 2000, UK).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching
O stretching
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe) − k1t
qe) − k1t
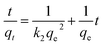
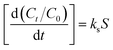

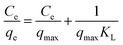
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + 1/n
KF + 1/n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce