Open Access Article
Open Access ArticleBall milling for the quantitative and specific solvent-free Knoevenagel condensation + Michael addition cascade in the synthesis of various 2-amino-4-aryl-3-cyano-4H-chromenes without heating†
Omid Hosseinchi
Qareaghaj
a,
Sara
Mashkouri
a,
M. Reza
Naimi-Jamal
*a and
Gerd
Kaupp
b
aResearch Laboratory of Green Organic Synthesis and Polymers, Department of Chemistry, Iran University of Science and Technology, 16846 Tehran, Iran. E-mail: naimi@iust.ac.ir
bFaculty 5, Department of Chemistry, University of Oldenburg, 26188 Edewecht, Germany
First published on 11th September 2014
Abstract
Ball milling is used as a facile, efficient, cheap, and environmentally friendly procedure for the solvent-free three-component reaction of aromatic aldehydes with malononitrile and dimedone, or 1,3-cyclohexanedione, resorcinol, and α- and β-naphthol. The reactions proceed quantitatively at room temperature by milling stoichiometric mixtures of the reagents in the presence of 10 mol% Na2CO3. This method offers the advantages of short reaction times, low cost, quantitative yields and simple work-up with no need of any organic solvent. It is thus enviro-economic without producing dangerous wastes. The orientation selectivity with β-naphthol and resorcinol is discussed. Existing structural misattributions are pointed out and clarified by 1H NMR spin coupling analysis.
Introduction
The versatile synthesis of 2-amino-3-cyano-4-aryl-4H-chromenes is presently one of the most studied vehicles for the presentation of new catalysts with mostly weak acids and bases, even though uncatalyzed reactions of aldehydes with malononitrile and 1,3-diketones provide quantitative wasteless yields in stoichiometric solventless melt-reactions at still moderate temperatures (125–150 °C). Clearly, the uncatalyzed syntheses provide the best possible scale-up possibilities,1 but also enviro-economic catalysis by milling with soda at room temperature is of interest for reactions with less reactive reagents.2 The general importance of chromene derivatives or amino-4H-chromenes has been amply reported in ref. 3 and in the numerous papers cited therein, but we focus here on the importance of 2-amino-3-cyano-4-aryl-4H-chromenes. These compounds have been identified as having the capacity to prevent various types of human diseases.4,5 These include antiallergic,6 antitumor7–9 and antibacterial activities.10 Most recent interest has focused on these compounds with additional substituents for anti-microbial activities in pharmaceutical applications and biological studies.11,12 Furthermore, the 2-amino-4-aryl-3-cyano-substituted 4H-chromenes are being used as building blocks for the synthesis of human drugs for many kinds of neurodegenerative and other serious diseases.13,14 For example, most 4H-chromene derivatives with particular substituents show antibacterial activity even against highly resistant Staphylococcus aureus and others.15 Several of these compounds are thus already commercially available. Importantly, also both the 2-amino and the 3-cyano substituents have been variously converted with standard transformations to create a multitude of compounds with useful applications. But unfortunately there is some confusion as to the correct chemical structure of these very important commercial compounds, because the necessary corrections of unduly claimed orientation selectivities in some of the multiple component reactions (MCRs) were never performed, and the reasons for the selectivities were not discussed.The synthesis by Knoevenagel condensation + Michael addition of the highly substituted 4H-chromenes has been varied by using many methods in recent years in an attempt to improve ease and efficiency by catalysis. The two-step approach by cyclization of preformed arylidenemalononitriles with β-dicarbonyl compounds is accelerated by the presence of an organic base such as piperidine,16 morpholine, pyridine,17 and triethylamine.18,19 Furthermore, despite our report of an early quantitative wasteless procedure for stoichiometric melt reactions without catalyst,1 further two-step procedures have been tried to decrease the temperature by use of a catalyst at the expense of lower yield and the need for chromatographic workup.20–37 This is also true for the later tried MCRs for the preparation of 4H-chromenes. The catalytic processes for the one-pot synthesis of 2-amino-4-aryl-3-cyano-substituted 4H-chromenes have been recently complemented by the application of potassium phthalimido-N-hydroxylate in water as another base though by use of 10% excess of the reagent malononitrile,3 which however creates additional wastes when recycling the catalyst. Other approaches utilized for example hexadecyltrimethylammonium bromide (HTMAB),20 sodium stearate,21 4-dodecylbenzenesulfonic acid (DBSA),20 KF–Al2O3,22 the rare earth perfluorooctanoate (Re(PFO)3),23 (S)-proline,24 (NH4)2HPO4,25 NMe4OH,26 MgO,27,28 tetrabutylammonium fluoride (TBAF),29 CeCl3·7H2O,30 ZnO-beta-zeolite,31 1,4-diazabicyclo[2.2.2]octane (DABCO),32 silica gel-supported polyphosphoric acid (PPA–SiO2),33γ-Fe2O3,34 Caro's acid silica gel,35 and amino-functionalized ionic liquids.36 Some of these methods applied organic solvent such as ethanol, dimethylformamide (DMF) or acetic acid,24,37 others water with propylpiperazine-N-sulfonic acid,38 or hydrocalcite,39 or 1-deoxy-1-methylaminosorbitol.40 Also, heating by microwave41 or ultrasonic irradiation42 was applied. However, most of these procedures have drawbacks of producing dangerous wastes or providing less than quantitative yield of pure product. These are as yet only overcome upon stoichiometric heating of the components at 150 °C and 100–130 °C without catalyst.1 Some catalysis protocols still require elevated temperatures, often hazardous organic solvents, and organic bases. Furthermore, the catalysts are often expensive or not commercially available, and tedious workup procedures are the rule with many wastes, because of only moderate product yields, requiring chromatographic workup and recrystallization. Thus, quantitative yield of the target products at room temperature omitting wasteful workup is highly desired. We therefore applied the usefulness of the solventless (solvent-free when choosing aqueous workup) stoichiometric MCR technique with the easily removable environmentally safe catalyst sodium carbonate in a ball-mill,2,43–47 and keep in mind the possibility that ball milling is scalable to hundreds of grams,48–52 which contrasts with recent reviews on milling in organic synthesis with complaints of lack of scale-up developments.53 But the existence of the reviews48–52 on such scale-up (further applications in ref. 48–52) has been pointed out.54 We have already reported that combining MCRs and ball milling allows for the synthesis of a wide variety of interesting organic building blocks.43–52,55,56 We also reported that sodium carbonate can be a useful basic catalyst in kneading milling.44–47 This technique benefits from both the economical and the synthetic point of view by not only omitting the unnecessary use of organic solvents, but also enhancing the rate of many organic reactions.
The ongoing hype with multiple repetitions of the MCR syntheses in question, providing high melting-point products, bears the risk that the characterization of the products rests solely on melting point comparison rather than on interpretation of 1H NMR coupling schemes that must fit with the claimed orientation specificities in these reactions. Unfortunately, there are never-resolved discrepancies in the claimed molecular structures when for example β-naphthol or resorcinol was the reagent for Michael additions followed by cyclization, and one must not invoke melting points of published unassigned or merely claimed molecular structures without considering a safe structure elucidation.
With the desire to understand the surprising orientation specificities in these MCR syntheses, we continued our preliminary grinding and heating experiments2 by ball milling with sodium carbonate catalysis in order to obtain quantitative yields without heating and without producing dangerous wastes. We assess the correctness of the product structures and report on the selectivity of the weakly basic catalyst towards phenols. These one-pot solvent-free syntheses react various benzaldehydes with malononitrile and dimedone, or 1,3-cyclohexandione, or resorcinol, or α- or β-naphthol. Quantitative yields are indeed obtained by kneading ball milling with sodium carbonate as an efficient, selective, cheap, enviro-economic, and easily separated catalyst without the need for external heating.
Results
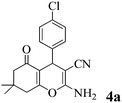
It was previously reported that the catalyst-free ball-milling reaction of 4-chlorobenzaldehyde (1, Ar = 4-ClC6H4) with malononitrile (2) to give the Knoevenagel condensate could not be completed in the solid state after 10 min at room temperature but afforded only 7% of the corresponding benzylidene malononitrile 9 (Scheme 1).57 However, the uncatalyzed melt reactions occurred quantitatively at 150 °C with 4-H-, 4-OH-, 4-Cl- and 4-NO2-benzaldehyde and the stoichiometric follow-up Michael additions with dimedone (3a) occurred in melts at 100–130 °C to quantitatively give the 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydro-4H-chromenes 4a.1 The one-pot MCR of 4-chlorobenzaldehyde (1a) with malononitrile (2) and less reactive β-naphthol (3e) was incomplete (78% 8a) at 150 °C (4 h), but when 10 mol% of ground Na2CO3 was admixed, the reaction temperature could be decreased to 125 °C (1 h) and quantitative or near-quantitative yields were obtained also with a series of further benzaldehydes (Scheme 1).2We now found that a quantitative yield of the product 8a (Table 1) was obtained at room temperature when the above mixture was milled for 10 min. 100% conversion was achieved because the product becomes an insoluble powder. After sublimation in a vacuum at 180 °C the product was completely separated from the recycled catalyst and this process was thus “solventless”. But such high workup temperature could be avoided by simple extraction of the catalyst from the insoluble product crystals with water in a then “solvent-free” process. No extra purifying workup was required in both techniques.
| Entry | Aldehyde (1) | Enol-naphthol | Product | Time (min) | Yielda (%) | M.p. (obsd.) (°C) | M.p. (lit.) (°C) |
|---|---|---|---|---|---|---|---|
| a Isolated yields after extraction of Na2CO3 from the insoluble crystals with water and drying. b For the quantitative yield for this case, subsequent heating of the reaction mixture at 125 °C was necessary. | |||||||
| 1 | 4-Cl-C6H4CHO | 3a |
|
10 | >99 | 213–214 | (208–210)17 |
| 2 | PhCHO | 3a |
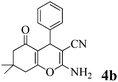
|
25 | >99 | 219–221 | (226–228)10 |
| 3 | 2-Cl-C6H4CHO | 3a |
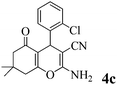
|
15 | >99 | 196–197 | (191–192)25 |
| 4 | 4-Me-C6H4CHO | 3a |
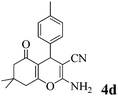
|
35 | >99 | 211–213 | (210–213)23 |
| 5 | 4-NO2-C6H4CHO | 3a |
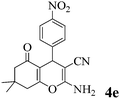
|
10 | >99 | 177–178 | (177–179)23 |
| 6 | 3-NO2-C6H4CHO | 3a |
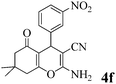
|
20 | >99 | 206–209 | (204–205)23 |
| 7 | 4-CN-C6H4CHO | 3a |
|
10 | >99 | 221–224 | (225–228)23 |
| 8 | 4-Br-C6H4CHO | 3a |
|
35 | >99 | 196–198 | (196–198)69 |
| 9 | 4-MeO-C6H4CHO | 3a |
|
35 | >99 | 197–199 | (201–202)25 |
| 10 | 4-HO-C6H4CHO | 3a |
|
40 | >99 | 205–206 | (204–205)25 |
| 11 | 2-NO2-C6H4CHO | 3a |
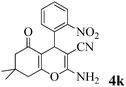
|
15 | >99 | 220–221 | (233–234)25 |
| 12 | 4-Cl-C6H4CHO | 3b |
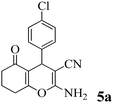
|
20 | >99 | 241–244 | (241–243)70 |
| 13 | 3-NO2-C6H4CHO | 3b |
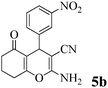
|
20 | >99 | 229–230 | (221–223)71 |
| 14 | 4-NO2-C6H4CHO | 3b |
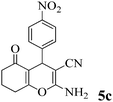
|
10 | >99 | 223–225 | (234–236)72 |
| 15 | 4-Br-C6H4CHO | 3b |
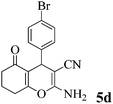
|
35 | >99 | 238–240 | (241–242)73 |
| 16 | 4-HO-C6H4CHO | 3b |
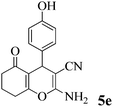
|
40 | >99 | 244–246 | (234–236)72 |
| 17 | 2-Cl-C6H4CHO | 3b |
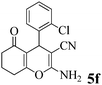
|
15 | >99 | 205–208 | (208–210)74 |
| 18 | 4-MeO-C6H4CHO | 3b |
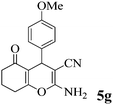
|
35 | >99 | 199–202 | (198–200)75 |
| 19 | PhCHO | 3c |
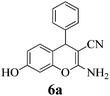
|
10 | >99 | 230–231 | (230–232)3 |
| 20 | 4-Cl-C6H4CHO | 3c |
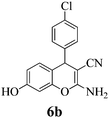
|
5 | >99 | 228–230 | (230–231)76 |
| 21 | 4-Br-C6H4CHO | 3c |
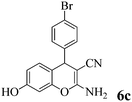
|
5 | >99 | 240–241 | (240–241)77 |
| 22 | 4-Cl-C6H4CHO | 3d |
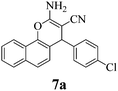
|
10 | >99 | 233–235 | (233–235)3 |
| 23 | 4-Br-C6H4CHO | 3d |
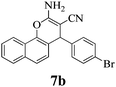
|
10 | >99 | 232–234 | (234–236)3 |
| 24 | 4-Cl-C6H4CHO | 3e |
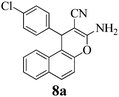
|
12 | >99 | 207–209 | (207–208)3 |
| 25 | 3-NO2-C6H4CHO | 3e |
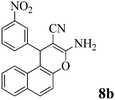
|
12 | >99 | 237–239 | (232–235)2 |
| 26 | 4-HO-C6H4CHO | 3e |
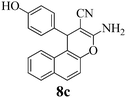
|
30b | >99 | 260–262 | (260–262)2 |
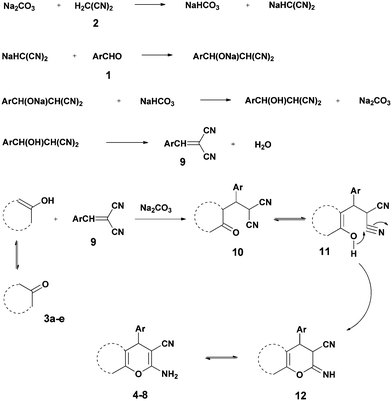
Kneading in the presence of sodium carbonate can now be used to provide the completion of both the Knoevenagel condensations by addition of aldehydes 1 with malononitrile 2 (m.p. 32 °C) followed by dehydration to give 9 and the Michael addition with 1,3-diketones 3a and 3b (as their enols) or with the reactive phenols 3c–e in the 25–30 °C range. Mechanistically, the intermediates 10, which are in equilibrium with their enols 11, undergo intramolecular cyclization to give 12, the tautomers of the products 4–8.1 Apparently, the basicity of ball-milled Na2CO3 and the acidity of NaHCO3 are improved by continued creation of new surfaces of these solids (Scheme 1).
The corresponding results with dimedone (3a), 1,3-cyclohexanedione (3b), resorcinol (3c), and α- (3d) and β-naphthol (3b) (Scheme 2) are reported in Table 1. The times for quantitative reaction were determined by thin layer chromatography (TLC) tests. Table 1 reveals that electron-withdrawing groups accelerate the reaction. For example, all reactions of 4-nitro- and 4-cyanobenzaldehyde and 2 with 3a–3e were completed after 10 minutes milling, but 4-methoxy- and 4-hydroxybenzaldehyde needed up to 40 minutes for completion. Also the ball milling of 4-chloro- and 4-bromobenzaldehydes proceeded rapidly and quantitatively without external heating. Only in the case of 8c was the subsequent heating of the reaction mixture to 125 °C for 1 h necessary to afford the product quantitatively, probably due to the presence of the electron-releasing phenolic OH group and the least reactivity of β-naphthol among the other reagents 3. Conversely, all uncatalyzed quantitative corresponding reactions required heating to 125 °C for 1 h.2 Solid Na2CO3 has a clear advantage over K2CO3. In similar work, Zhou et al.58 reported lower yields of 67–74% when grinding with stoichiometric amounts of K2CO3 and could not avoid the formation of deeply colored by-products. A major difference appears to be the different qualities of the continuously hydrated catalysts when the water of reaction is liberated. It is well known that sodium carbonate takes up 10H2O to form a stable solid hydrate, whereas the stable solid hydrate of potassium carbonate contains only 1.5H2O. Also stoichiometric grinding with the weaker basic NaHCO3 was inferior in that it gave 74–93% yield upon similar grinding for 90 min, here without coloration.58
The surprising orientation specificities giving only one product in all cases were determined by purity checks with sharp melting points, TLC and the physical data that also correspond to the available published ones. The NMR spectroscopic data reveal only one series of resonance signals.
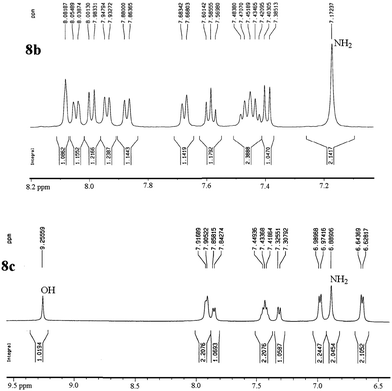
The structures of all products were obtained on the basis of their spectral data. The typical NH2 and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N FT-IR bands are of course present in the spectra of all of the corresponding derivatives. The 1H NMR spectra of all described products show one characteristic singlet signal in the range of 4.1–5.6 ppm for the 4-H and the 13C NMR spectra exhibit one specific signal in the range of 54–60 ppm for the carbon at position 4. The reaction orientation of the products 6 and 8 is still controversial. Thus, in a series of papers, Kumar59–61 published the reactions of β-naphthol with aromatic aldehydes and malononitrile using various catalysts and heating devices including microwaves as giving straight “2-amino-4-aryl-3-cyano-4H-benzo[g]chromenes” (from claimed reaction at C-3 of β-naphthol), rather than the angular 2-amino-4-aryl-3-cyano-4H-benzo[f]chromenes (reaction at C-1 of β-naphthol), as we do and many other research groups in the field. The same discrepancy exists when cyanoaceticethylester is used instead of malononitrile for providing the corresponding products with 3-ethoxycarbonyl substitution. Furthermore, the CAS Registry Database also interprets the poorly defined products with β-naphthol in the papers of Pasha62 and Jin63 with the straight ring condensation. A clear discussion of the correct structure of the products of these MCRs is of utmost importance, as several of these products are commercially available. For example the product from benzaldehyde, malononitrile and β-naphthol is still sold and advertised (December 2013 according to CAS Registry Database) by 4 companies (American Custom Chemical Corp.; ChemPur GmbH; Fluorochem Ltd; Gencore Bio PharmaPvT Ltd) as straight “-4H-naphtho[2,3-b]pyran” (a synonym for “-4H-benzo[g]chromene”) (CAS registry number 924708-55-4). Similar advertisements continue to exist with substituted products with claimed straight ring condensation. Conversely, 23 suppliers sell these products as angular “-4-H-naphtho[2,1-b]-pyran” (a synonym for -4H-benzo[f]chromene) (CAS registry number 111861-46-2 for Ar = C6H5). As the research groups that published the angular structure (reaction at C-1 of β-naphthol) also used various catalysts (e.g. TiCl4 or bases or phase-transfer catalysis) at room temperature or with heating (including microwave), it appears clear that one of these orientation claims must be wrong. Clearly, high-resolution 1H NMR provides a clear decision. Unfortunately, the claims of the straight structure were never substantiated by a crystal structure determination or by an analysis or discussion of the aromatic proton resonance coupling schemes by 1H NMR. Thus these papers59–63 do not account for the Ar–H resonances that are only published as multiplet containing 10 or 11 Ar–H. However, our 1H NMR spectra2 exhibit the Ar–H signal at highest field for all substituted derivatives of the β-naphthol products with large splitting (7–9 Hz range of an AB spin system) evidently by the coupling with the vicinal Ar–H, and so do our here studied products (see the examples 8b and 8c in Fig. 1 with the analysis in the Experimental section).
N FT-IR bands are of course present in the spectra of all of the corresponding derivatives. The 1H NMR spectra of all described products show one characteristic singlet signal in the range of 4.1–5.6 ppm for the 4-H and the 13C NMR spectra exhibit one specific signal in the range of 54–60 ppm for the carbon at position 4. The reaction orientation of the products 6 and 8 is still controversial. Thus, in a series of papers, Kumar59–61 published the reactions of β-naphthol with aromatic aldehydes and malononitrile using various catalysts and heating devices including microwaves as giving straight “2-amino-4-aryl-3-cyano-4H-benzo[g]chromenes” (from claimed reaction at C-3 of β-naphthol), rather than the angular 2-amino-4-aryl-3-cyano-4H-benzo[f]chromenes (reaction at C-1 of β-naphthol), as we do and many other research groups in the field. The same discrepancy exists when cyanoaceticethylester is used instead of malononitrile for providing the corresponding products with 3-ethoxycarbonyl substitution. Furthermore, the CAS Registry Database also interprets the poorly defined products with β-naphthol in the papers of Pasha62 and Jin63 with the straight ring condensation. A clear discussion of the correct structure of the products of these MCRs is of utmost importance, as several of these products are commercially available. For example the product from benzaldehyde, malononitrile and β-naphthol is still sold and advertised (December 2013 according to CAS Registry Database) by 4 companies (American Custom Chemical Corp.; ChemPur GmbH; Fluorochem Ltd; Gencore Bio PharmaPvT Ltd) as straight “-4H-naphtho[2,3-b]pyran” (a synonym for “-4H-benzo[g]chromene”) (CAS registry number 924708-55-4). Similar advertisements continue to exist with substituted products with claimed straight ring condensation. Conversely, 23 suppliers sell these products as angular “-4-H-naphtho[2,1-b]-pyran” (a synonym for -4H-benzo[f]chromene) (CAS registry number 111861-46-2 for Ar = C6H5). As the research groups that published the angular structure (reaction at C-1 of β-naphthol) also used various catalysts (e.g. TiCl4 or bases or phase-transfer catalysis) at room temperature or with heating (including microwave), it appears clear that one of these orientation claims must be wrong. Clearly, high-resolution 1H NMR provides a clear decision. Unfortunately, the claims of the straight structure were never substantiated by a crystal structure determination or by an analysis or discussion of the aromatic proton resonance coupling schemes by 1H NMR. Thus these papers59–63 do not account for the Ar–H resonances that are only published as multiplet containing 10 or 11 Ar–H. However, our 1H NMR spectra2 exhibit the Ar–H signal at highest field for all substituted derivatives of the β-naphthol products with large splitting (7–9 Hz range of an AB spin system) evidently by the coupling with the vicinal Ar–H, and so do our here studied products (see the examples 8b and 8c in Fig. 1 with the analysis in the Experimental section).
Also when checking the corresponding 1H NMR data of others, who disclosed the spectra (though without discussion), one finds the same coupling scheme. There cannot be any doubt that the Ar–H signal at highest field belongs to the hydrogen that is next to the heterocyclic oxygen. Furthermore, two slightly broadened singlet signals would have to be expected from the Ar–H moieties of the central ring in the straight structure. But such singlets cannot be found in any of these spectra. This now safely validates the angular -4H-benzo[f]chromene structures of the products from the reactions with β-naphthol, independent of the reaction conditions and arylic substitution. Also, published X-ray diffraction analyses of related compounds (8a with H,64 CH3,65 or Br (ref. 66) instead of Cl, the latter in the (S)-configuration) support the angular structure. Clearly, all chemical, biological, and medicinal results that were based on the still commercially advertised and sold products with the unsupported nominal straight “-4H-”benzo[g]chromene structure must be urgently reinterpreted.
Another structural question to be settled is the surprising orientation specificity of the reactions with resorcinol. While a reaction at its C-2 position would be electronically more probable, the greater crowding of the aryl group with the remaining OH group would disfavor that possibility. Thus, reaction at C-4 of resorcinol comes to the fore. Experimentally, one obtains the 7-hydroxy-4H-chromene structures 6 but not the 5-OH-4H-chromene isomers, not even as detectable side-products. Unfortunately, we cannot argue with product stabilities because quantum chemical calculations predict the actually obtained 6a and the not obtained isomeric structure are energetically very close. The semi-empirical PM3 method calculates the obtained 7-OH structure as being 0.28 kcal mol−1 more stable, but DFT B3LYP 6-31G* calculates the not obtained 5-OH structure as being 0.015 kcal mol−1 more stable. The 1H NMR spectra of 6a–6c provide a clear structural decision. They exhibit the high-field Ar–H (the 8-H that is next to the heterocyclic oxygen atom) as slightly broadened singlet signal (with unresolved 4J or 5J long-range couplings below 1.8 Hz or smaller), and a 6-H/5-H AB system (the 6-H signal with more long-range broadening), in addition to the deeper field Ar–H signals of the 4-substituent (AA′BB′ for 6b and 6c). Thus, the structure 6 (reaction at C-4 of resorcinol as proposed in ref. 67) is confirmed, and the suggestion in ref. 68 (claiming reaction at C-2 of resorcinol) is unsupported. Importantly, our 1H NMR spectra of 6a–6c concur with the ones of ref. 13 also with the singlet signal from 8-H without vicinal coupling partner. These products were synthesized in solution with a “tungstic acid functionalized mesoporous catalyst”. Furthermore, the melting points of the corresponding products from refluxing water with potassium phthalimido-N-hydroxylate3 and those in ref. 76 and 77 correspond with ours. Thus, the structure 6 is found independent of the reaction conditions.
The remaining 7-OH group of 6a–6c was not able to undergo the Michael addition with excess arylidenemalononitriles under our reaction conditions. The electronic conditions would allow for it, but the acidity of 7-OH in the compounds 6 is apparently not high enough. Stronger conditions should be helpful for the corresponding second Michael addition.
The advantage of the present enviro-economic methodology may for example be demonstrated by comparison of the efficiencies for the syntheses of compound 4e in various previous publications (Table 2). The reported results clearly show that the present protocol at room temperature and with short reaction times is the only one giving quantitative yields and not requiring the use of organic solvents etc. for workup. The previous catalytic techniques sometimes at elevated temperatures and with long reaction times exhibit lower and never quantitative yields. They require costly purification workup with serious waste formation by removal/recovery of catalysts and organic non-product materials in organic solvents. It is also noteworthy that our easily separated catalyst, Na2CO3, is the cheapest and greenest one among those being compared. Our work-up procedure is extremely simple. The obtained solid product powder is quantitatively flushed out with water from the milling vessel, filtered and dried for quantitatively isolated pure product yields and a soda solution. No purification workup was necessary. These solvent-free syntheses are thus enviro-economic. When desired, the powders can also be directly sublimed off from the catalyst.
| Catalyst | Temp. | Time | Catalyst loading | Yield (%) |
|---|---|---|---|---|
| This work (Na2CO3) | rt | 10 min | 10 mol% | 100 |
| Re(PFO)3 (ref. 23) | 60 °C | 4 h | 5 mol% | 90 |
| Diammonium hydrogen phosphate25 | rt | 0.5–2 h | 10 mol% | 78 |
| NMe4OH (ref. 26) | rt | 0.5–2 h | 10 mol% | 80 |
| CeCl3 (ref. 30) | Reflux | 1 h | 10 mol% | 93 |
| ZnO-beta zeolite31 | Reflux | 50 min | 100 mg | 87 |
| DABCO32 | Reflux | 2 h | 10 mol% | 94 |
| Silica gel-supported polyphosphoric acid33 | Reflux | 8 min | 100 mg | 85 |
| Caro's acid–silica gel35 | Reflux | 15 min | 16 mol% | 93 |
| D-L-Proline24 | rt | >30 min | 20 mol% | 92 |
Discussion
The selectivity of the milled solid catalyst Na2CO3 between different phenols is remarkable. The reactions go to completion despite the increasing amounts of water of crystallization from the Knoevenagel condensation. Since the solid decahydrate of soda (m.p. 34 °C) might have easily melted in its environment, it is almost certain that this hydration state was not reached and that at least part of the water of reaction was also included in the solid products with many functional groups. While resorcinol and the naphthols react smoothly, phenol and the phenolic groups in 4j or 6a–6c do not react with the Knoevenagel reagents 9. This may be roughly related to the well-known pKa values for aqueous solution even though the acidities in the present medium would be different. Thus, the reactive phenols such as resorcinol (pKa1 = 9.15), α-naphthol (pKa = 9.3), and β-naphthol (pKa = 9.57) are considerably stronger acids than the weaker alkyl- and alkoxyphenols. We may refer here to phenol (pKa = 9.99) and reference compounds such as 4-methylphenol (pKa = 10.26) and 2-methoxyphenol (pKa = 9.98). It thus appears that our solid catalyst cannot deprotonate the weaker phenols 4j and 6a–6c and this would explain its selection between phenols.Also the exclusive Michael addition of β-naphthol at C-1 is highly remarkable, because reaction at C-3 would be facilitated by less steric crowding. It appears, however, that the much higher electron density of the naphthol and of the naphtholate at C-1 is the prevailing factor. Density functional calculations at the B3LYP 6-31G* level calculate the electrostatic charges at C-1 as −0.505 and at C-3 as −0.224 for β-naphthol or as −0.605 at C-1 and as −0.326 at C-3 for the β-naphtholate anion. The large electrostatic differences substantiate the experimentally found orientation specificities to C-1 but not to C-3 of β-naphthol in its reactions with the Knoevenagel intermediate 9. Kumar's group unduly claimed the latter choice without analysis of their 1H NMR data.59–61
To exclude the possibility that their experimental technique would have drastically changed the orientation specificity of β-naphthol, the reported melting points in ref. 60–62 are invoked. The now challenged “2-amino-4-aryl-3-cyano-4H-benzo[g]chromenes” are reported with melting points of 274, 187, 191, and 260 °C for Ar = 4-H, 4-NO2, 4-CH3O, and 2-Cl-phenyl.59–61 These values concur with those of the 1H NMR spectroscopically validated 2-amino-4-aryl-3-cyano-4H-benzo[f]chromenes. Furthermore, the melting points correspond with those of all other published -4H-benzo[f]chromenes that consistently exhibit the splitting of the highest field Ar–H signal in the 1H NMR spectrum from the cited authors in Table 1 and also in Table 2 of ref. 2. Thus β-naphthol reacts at C-1 but not at C-3 independent of the experimental conditions, because the electron density is almost twice as high at the experimentally chosen position.
Conclusions and outlook
We have demonstrated a “green” atom-economic and enviro-economic method for the solid-state preparation of several 2-amino-4-aryl-3-cyano-4H-chromenes and clarified still existing detrimental controversies concerning the product structures. The solvent-free technique with the eco-efficient catalyst is superior to the less productive solution techniques that use expensive and harmful catalysts, solid supports, ultrasound or microwave, which require purifying workup by using organic solvents for removal of the support, of unreacted reagents, and of side products. The here reported milling protocol without heating has the advantages of operational simplicity, mild reaction conditions, quantitative product yields, and no need of organic solvents for workup and purification. The soda catalyst is cheap and non-toxic. These benefits make our methodology a valid contribution to the existing processes in the field of 2-amino-4-aryl-3-cyano-4H-chromene derivative synthesis.Experimental section
Material and methods
The ball mill was a Retsch MM 400 swing mill. A 10 mL stainless steel vessel was used. Two stainless steel balls with 7 mm diameter were used, and the milling frequency was 30–35 Hz. The temperature within the milling beaker was close to room temperature of about 25 °C. It never reached or exceeded 30 °C by the milling, but malononitrile (m.p. 32 °C) melts in the presence of the other reactants, so this is actually kneading. Melting points were determined with a Barnstead electrothermal melting point apparatus and are uncorrected. Infrared (IR) spectra were recorded with a Shimadzu 8400s FT-IR spectrometer using potassium bromide pellets. 500 MHz 1H NMR spectra were recorded with a Bruker DRX-500 Avance spectrometer and 250 MHz spectra with a Bruker Avance DPX-250. The chemical shifts are reported in ppm (δ-scale) relative to internal TMS and coupling constants are reported in DMSO-d6. Density functional calculations were performed with the TITAN program of Wave Function Inc., Irvine, USA and Schrödinger Inc., Portland, USA. All solid reagents were purchased from commercial sources and used without further purification when uniformity was tested by TLC (silica gel with ethyl acetate and hexane in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio). The liquid aldehydes were distilled prior to use. All products are previously described compounds and they were identified and structurally characterized. Their physical and spectral data corresponded with the cited literature data. Product purity was checked by melting point, by TLC on silica gel with ethyl acetate and hexane in a 1
5 ratio). The liquid aldehydes were distilled prior to use. All products are previously described compounds and they were identified and structurally characterized. Their physical and spectral data corresponded with the cited literature data. Product purity was checked by melting point, by TLC on silica gel with ethyl acetate and hexane in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, and by high-resolution 1H NMR spectra.
5 ratio, and by high-resolution 1H NMR spectra.
Typical procedure for the synthesis of the 4H-chromenes
In a typical experiment, a stoichiometric mixture of an aromatic aldehyde (1) (1.0 mmol), malononitrile (2) (66 mg, 1.0 mmol), dimedone (3a) (140 mg, 1.0 mmol) or 3b–3e (112 mg, 110 mg, 144 mg, and 144 mg, respectively, 1.0 mmol each) and anhydrous sodium carbonate (11 mg, 0.1 mmol) were ball-milled for the appropriate time (Table 1). When the reaction was completed, as indicated by the FTIR spectrum of the solid in KBr pellets and TLC to detect uniformity, the reaction product (from repeat runs without taking material for IR and TLC) was scraped out on a filter and the holdup flushed out with cold washing water (3 times, 5 mL each) that was shaken in the mill for about 1 min prior to pouring on the solid product for extracting the inorganic catalyst. After drying the product from the filter in a vacuum, the pure product was directly obtained with no need for extra purification. The products could also be quantitatively obtained in pure form by complete collection from the ball-milling vessel (using the holdup from a previous run) and sublimation in a good vacuum in the temperature range of 150–190 °C. FTIR spectra of the washed or recrystallized or sublimed products corresponded to the raw spectra still containing the catalyst.Representative spectral data
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CN), 115.47, 117.74, 121.04, 122.18, 122.72, 124.36, 128.28, 129.48, 130.78, 130.95, 131.33 (2C), 131.73 (2C), 134.59, 148.61, 148.86, 160.84 (
C–CN), 115.47, 117.74, 121.04, 122.18, 122.72, 124.36, 128.28, 129.48, 130.78, 130.95, 131.33 (2C), 131.73 (2C), 134.59, 148.61, 148.86, 160.84 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, –N).
C–O, –N).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CN), 116.21 (2C), 117.04, 117.65, 121.51, 124.59, 125.71, 127.81, 128.88 (2C), 129.28, 130.10, 131.09, 131.68, 137.11, 147.54, 156.82, 160.38 (
C–CN), 116.21 (2C), 117.04, 117.65, 121.51, 124.59, 125.71, 127.81, 128.88 (2C), 129.28, 130.10, 131.09, 131.68, 137.11, 147.54, 156.82, 160.38 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, –N).
C–O, –N).
The ESI† contains typical prints of the recorded NMR and IR spectra. It can be accessed free of charge.
Acknowledgements
The financial support from The Research Council of Iran University of Science and Technology (IUST), Tehran, Iran is gratefully acknowledged.Notes and references
- G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Tetrahedron, 2003, 59, 3753–3760 CrossRef CAS.
- M. R. Naimi-Jamal, S. Mashkouri and A. Sharifi, Mol. Diversity, 2010, 14, 473–477 CrossRef CAS PubMed.
- M. G. Dekamin, M. Eslami and A. Maleki, Tetrahedron, 2013, 69, 1074–1085 CrossRef CAS PubMed.
- L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517–520 CrossRef CAS.
- Y. Gao, W. Yang and D.-M. Du, Tetrahedron: Asymmetry, 2012, 23, 339–344 CrossRef CAS PubMed.
- R. Ling, M. Yoshida and P. S. Mariano, J. Org. Chem., 1996, 61, 4439–4449 CrossRef CAS PubMed.
- L. R. Morgan, B. S. Jursic, C. L. Hooper, D. M. Neumann, K. Thangaraj and B. LeBlanc, Bioorg. Med. Chem. Lett., 2002, 12, 3407–3411 CrossRef CAS.
- A. Bolognese, G. Correale, M. Manfra, A. Lavecchia, O. Mazzoni, E. Novellino, P. La Colla, G. Sanna and R. Loddo, J. Med. Chem., 2004, 47, 849–858 CrossRef CAS PubMed.
- H. Gourdeau, L. Leblond, B. Hamelin, C. Desputeau, K. Dong, I. Kianicka, D. Custeau, C. Boudreau, L. Geerts, S.-X. Cai, J. Drewe, D. Labrecque, S. Kasibhatla and B. Tseng, Mol. Cancer Ther., 2004, 3, 1375–1384 CAS.
- N. Fokialakis, P. Magiatis, I. Chinou, S. Mitaku and F. Tillequin, Chem. Pharm. Bull., 2002, 50, 413–414 CrossRef CAS.
- A. Afantitis, G. Melagraki, H. Sarimveis, P. A. Koutentis, J. Markopoulos and O. Igglessi-Markopoulou, Bioorg. Med. Chem., 2006, 14, 6686–6694 CrossRef CAS PubMed.
- M. H. Fatemi and S. Gharaghani, Bioorg. Med. Chem., 2007, 15, 7746–7754 CrossRef CAS PubMed.
- S. K. Kundu, J. Mondal and A. Bhaumik, Dalton Trans., 2013, 42, 10515–10524 RSC.
- C. S. Konkoy, D. B. Fick, S. X. Cai, N. C. Lan and J. F. W. Keana, Pct Int. Appl., WO 0075123A1, 2000 p. 1214; Chem. Abstr. 2001, 134, 29313a Search PubMed.
- M. Kidwai, S. Saxena, M. K. Rahman Khan and S. S. Thukral, Bioorg. Med. Chem. Lett., 2005, 15, 4295–4298 CrossRef CAS PubMed.
- N. Martin, C. Pascual, C. Seoane and J. Soto, Heterocycles, 1987, 26, 2811–2816 CrossRef CAS.
- A.-F. A. Harb, A.-H. M. Hesien, S. A. Metwally and M. H. Elnagdi, Liebigs Ann. Chem., 1989, 585–588 CrossRef CAS.
- S. E. Zayed, E. I. Abou Elmaged, S. A. Metwally and M. H. Elnagdi, Collect. Czech. Chem. Commun., 1991, 56, 2175–2182 CrossRef CAS.
- M. H. Elnagdi, R. M. Abdel-Motaleb, M. Mustafa, M. F. Zayed and E. M. Kamel, J. Heterocycl. Chem., 1987, 24, 1677–1681 CrossRef CAS.
- T. Jin, A. Wang, J. Zhang, F. Zhang and T. Li, Chin. J. Org. Chem., 2004, 24, 1598–1600 CAS.
- L.-M. Wang, N. Jiao, J. Qiu, J.-J. Yu, J.-Q. Liu, F.-L. Guo and Y. Liu, Tetrahedron, 2010, 66, 339–343 CrossRef CAS PubMed.
- X.-S. Wang, D.-Q. Shi, S.-J. Tu and C.-S. Yao, Synth. Commun., 2003, 33, 119–126 CrossRef CAS PubMed.
- L.-M. Wang, J.-H. Shao, H. Tian, Y.-H. Wang and B. Liu, J. Fluorine Chem., 2006, 127, 97–100 CrossRef CAS PubMed.
- S. Balalaie, M. Bararjanian, A. M. Amani and B. Movassagh, Synlett, 2006, 263–266 CrossRef CAS PubMed.
- S. Balalaie, M. Bararjanian, M. Sheikh-Ahmadi, S. Hekmat and P. Salehi, Synth. Commun., 2007, 37, 1097–1108 CrossRef CAS.
- S. Balalaie, M. Sheikh-Ahmadi and M. Bararjanian, Catal. Commun., 2007, 8, 1724–1728 CrossRef CAS PubMed.
- D. Kumar, V. B. Reddy, B. G. Mishra, R. K. Rana, M. N. Nadagouda and R. S. Varma, Tetrahedron, 2007, 63, 3093–3097 CrossRef CAS PubMed.
- M. Seifi and H. Sheibani, Catal. Lett., 2008, 126, 275–279 CrossRef CAS.
- S. Gao, C. Tseng, C. H. Tsai and C.-F. Yao, Tetrahedron, 2008, 64, 1955–1961 CrossRef CAS PubMed.
- G. Sabitha, K. Arundhathi, K. Sudhakar, B. Sastry and J. Yadav, Synth. Commun., 2009, 39, 2843–2851 CrossRef CAS.
- S. S. Katkar, M. K. Lande, B. R. Arbad and S. T. Gaikwad, Chin. J. Chem., 2011, 29, 199–202 CrossRef CAS.
- D. Tahmassebi, J. A. Bryson and S. I. Binz, Synth. Commun., 2011, 41, 2701–2711 CrossRef CAS.
- A. Davoodnia, S. Allameh, S. Fazli and N. Tavakoli-Hoseini, Chem. Pap., 2011, 65, 714–720 CrossRef CAS PubMed.
- S. Rostamnia, A. Nuri, H. Xin, A. Pourjavadi and S. H. Hosseini, Tetrahedron Lett., 2013, 54, 3344–3347 CrossRef CAS PubMed.
- H. A. Oskooie, M. M. Heravi, N. Karimi and M. E. Zadeh, Synth. Commun., 2011, 41, 436–440 CrossRef CAS.
- P. P. Salvi, A. M. Mandhare, A. S. Sartape, D. K. Pawar, S. H. Han and S. S. Kolekar, C. R. Chim., 2011, 14, 878–882 CrossRef CAS PubMed.
- K. Singh, J. Singh and H. Singh, Tetrahedron, 1996, 52, 14273–14280 CrossRef CAS.
- M. Ghashang, S. S. Mansoor and K. Aswin, Res. Chem. Intermed., 2013, 1–17, DOI:10.1007/s11164-013-1419-2.
- S. R. Kale, S. S. Kahandal, A. S. Burange, M. B. Gawande and R. V. Jayaram, Catal. Sci. Technol., 2013, 3, 2050–2056 CAS.
- R.-Y. Guo, Z.-M. An, L.-P. Mo, R.-Z. Wang, H.-X. Liu, S.-X. Wang and Z.-H. Zhang, ACS Comb. Sci., 2013, 15, 557–563 CrossRef CAS PubMed.
- J. Zhou, S. Tu, Y. Gao and M. Ji, Chin. J. Org. Chem., 2001, 21, 742–745 CAS.
- S. Tu, H. Jiang, Q. Zhuang, C. Miao, D. Shi, X. Wang and Y. Gao, Chin. J. Org. Chem., 2003, 23, 488–490 CAS.
- J. Mokhtari, M. R. Naimi-Jamal, H. Hamzeali, M. G. Dekamin and G. Kaupp, ChemSusChem, 2009, 2, 248–254 CrossRef CAS PubMed.
- S. Mashkouri and M. R. Naimi-Jamal, Molecules, 2009, 14, 474–479 CrossRef CAS PubMed.
- M. R. Naimi-Jamal, M. Mirzaei, M. Bolourtchian and A. Sharifi, Synth. Commun., 2006, 36, 2711–2717 CrossRef CAS.
- A. Sharifi, M. Mirzaei and M. R. Naimi-Jamal, J. Chem. Res., 2007, 129–132 CrossRef CAS.
- A. Sharifi, M. Mirzaei and M. R. Naimi-Jamal, Synth. Commun., 2005, 35, 1039–1044 CrossRef CAS PubMed.
- G. Kaupp, CrystEngComm, 2006, 8, 794–804 RSC.
- G. Kaupp, CrystEngComm, 2011, 13, 3108–3121 RSC.
- G. Kaupp, M. R. Naimi-Jamal and J. Schmeyers, Chem.–Eur. J., 2002, 8, 594–600 CrossRef CAS.
- G. Kaupp, in Encyclopedia of Life Support Systems (EOLSS), developed under the Auspices of the UNESCO, EOLSS Publishers, Oxford, UK, 2012, pp. 38, http://www.eolss.net Search PubMed.
- G. Kaupp, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2012, DOI:10.1002/0471238961.solvkaup.a01.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC.
- G. Kaupp and M. R. Naimi-Jamal, Eur. J. Org. Chem., 2002, 1368–1373 CrossRef CAS.
- G. Kaupp, M. R. Naimi-Jamal and V. Stepanenko, Chem.–Eur. J., 2003, 9, 4156–4161 CrossRef CAS PubMed.
- R. Trotzki, M. M. Hoffmann and B. Ondruschka, Green Chem., 2008, 10, 767–772 RSC.
- D. Zhou, Z. Ren, J. Chen, W. Cao and H. Deng, J. Heterocycl. Chem., 2008, 45, 1865–1867 CrossRef CAS.
- B. S. Kumar, N. Srinivasulu, R. H. Udupi, B. Rajitha, Y. T. Reddy, P. N. Reddy and P. S. Kumar, Russ. J. Org. Chem., 2006, 42, 1813–1815 CrossRef CAS.
- S. K. Kumar, M. S. Khalid and R. H. Udupi, Asian J. Chem., 2010, 22, 2685–2688 CAS.
- B. S. Kumar, N. Srinivasulu, R. H. Udupi, B. Rajitha, Y. T. Reddy, P. N. Reddy and P. S. Kumar, J. Heterocycl. Chem., 2006, 43, 1691–1693 CrossRef CAS.
- M. A. Pasha and V. Jayashankara, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2007, 46, 1328–1331 Search PubMed.
- T.-S. Jin, J.-C. Xiao, S.-J. Wang, T.-S. Li and X.-R. Song, Synlett, 2003, 2003, 2001–2004 CrossRef.
- M. Akkurt, A. R. Kennedy, S. K. Mohamed, S. H. H. Younes and G. J. Miller, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2013, 69, o401 CAS.
- P. Das, A. Dutta, A. Bhaumik and C. Mukhopadhyay, Green Chem., 2014, 16, 1426–1435 RSC.
- X.-S. Wang, G.-S. Yang and G. Zhao, Tetrahedron: Asymmetry, 2008, 19, 709–714 CrossRef CAS PubMed.
- V. J. Ram and M. Verma, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1994, 33, 908–911 Search PubMed.
- F. F. Abdel-Latif, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1990, 29, 664–666 Search PubMed.
- S. B. Guo, S. X. Wang and J. T. Li, Synth. Commun., 2007, 37, 2111–2120 CrossRef CAS.
- A. T. Khan, M. Lal, S. Ali and M. M. Khan, Tetrahedron Lett., 2011, 52, 5327–5332 CrossRef CAS PubMed.
- C. Revanna, T. Swaroop, G. Raghavendra, D. Bhadregowda, K. Mantelingu and K. Rangappa, J. Heterocycl. Chem., 2012, 49, 851–855 CrossRef CAS.
- J.-C. Xu, W.-M. Li, H. Zheng, Y.-F. Lai and P.-F. Zhang, Tetrahedron, 2011, 67, 9582–9587 CrossRef CAS PubMed.
- G. Zhang, Y. Zhang, J. Yan, R. Chen, S. Wang, Y. Ma and R. Wang, J. Org. Chem., 2011, 77, 878–888 CrossRef PubMed.
- H. R. Safaei, M. Shekouhy, S. Rahmanpur and A. Shirinfeshan, Green Chem., 2012, 14, 1696–1704 RSC.
- S. Nemouchi, R. Boulcina, B. Carboni and A. Debache, C. R. Chim., 2012, 15, 394–397 CrossRef CAS PubMed.
- A. M. El-Agrody, A. H. Bedair, T. C. Corkery, A. Ata and A. S. Abd-El-Aziz, Heterocycles, 2004, 63, 1793–1812 CrossRef.
- G. Klokol, L. Sharanina, V. Nesterov, V. Shklover, Y. Sharanin and Y. Struchkov, Zh. Org. Khim., 1987, 23, 412–421 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06603k |
| This journal is © The Royal Society of Chemistry 2014 |