DOI:
10.1039/C4RA00892H
(Paper)
RSC Adv., 2014,
4, 14320-14327
One-pot production of hydrocarbon oil from poly(3-hydroxybutyrate)†
Received
31st January 2014
, Accepted 7th March 2014
First published on 11th March 2014
Abstract
Poly(3-hydroxybutyrate) (PHB) is an energy storage material of many microbial species, and has been found to be an effective feedstock for production of renewable hydrocarbon oils. A high oil yield (up to 38.2 wt%) was obtained in a phosphoric acid (H3PO4) solution at mild temperatures (165–240 °C). PHB and crotonic acid (C4H6O2), a dominant thermal degradation product of PHB, were deoxygenated mainly via decarboxylation, generating similar liquid and gaseous products. Carbon dioxide and propylene were the major products in gas phase with little CO formation. The hydrocarbon oil (C4–C16) is a mixture of alkanes, alkenes, benzenes and naphthalenes. Aromatics (C10–C15) were the major hydrocarbons in a 100 wt% H3PO4 solution, while alkenes and alkanes (C4–C9) were favored in diluted solutions (50 wt% to 85 wt% H3PO4). The concentration of H3PO4 was a key factor that affected the oil composition and yield. A highly efficient decarboxylation of crotonic acid at 220 °C for 3 hours resulted in 70.8 wt% of oxygen being removed as CO2 and 57.0 wt% of carbon being recovered as hydrocarbon oil. The H3PO4 solution can be repeatedly used for high yield oil production. This work shows that a type of new biological feedstock can be used to produce renewable hydrocarbon oil in an efficient one-pot reaction.
1 Introduction
Poly(3-hydroxybutyrate) (PHB) is a biopolyester produced by many bacterial species as an energy storage material.1–3 It has been produced on large scales through industrial fermentation of renewable carbohydrates.4,5 PHB is an environmentally friendly bioplastic and has similar material properties to polypropylene for a variety of applications.6–8 PHB is also an energy-rich polymer and, similar to bio-oil, may be a renewable feedstock for production of liquid hydrocarbons. Little work, however, has been done to convert PHB into hydrocarbons as a “drop in” transportation fuel. Crotonic acid (trans-2 butenoic acid) is the major monomeric product formed from thermal degradation of PHB.9,10 Because of the presence of carboxyl groups, PHB deoxygenation is necessary in order to produce hydrocarbons. It has been reported that at high temperatures (>300 °C) propylene is formed from PHB decarboxylation in hydrothermal reforming.11 Lewis-acid may play a catalytic role in decarboxylation of unsaturated carboxylic acids at high temperature (365 °C).12 In general, hydrotreating and thermal (catalytic) pyrolysis are two of the most studied processes for deoxygenation of carboxylic compounds in biomass. Hydrotreating under high pressure hydrogen is a typical method to obtain hydrocarbons from bio-oils, but consumes a large amount of hydrogen.13,14 Thermal (catalytic) pyrolysis is an alternative processes for decarboxylation/decarbonylation of organic acids, which is usually performed at high temperatures (e.g., ≥300 °C).14–19 Thermogravimetric analysis has shown complete gasification of PHB at high temperatures (>280 °C),9,20,21 which implies that multiple catalytic reactors are needed to produce liquid hydrocarbons from a solid PHB-containing feedstock. Due to the worldwide demand for bio-based “drop in” transportation fuels, new renewable feedstocks such as PHB and processing technology should be explored. Specifically, a novel method for PHB deoxygenation at low temperature without hydrogen is meaningful.
Phosphoric acid (H3PO4) is usually considered as an indecomposable, non-volatile, non-oxidizing, mild acid, and has been used as a catalyst or solvent in many applications.22–26 In this work, we used H3PO4 solution in one-pot conversion of PHB into liquid hydrocarbon oil. It is first time demonstrated that a high oil yield is achieved at relatively low temperatures (165 to 240 °C) in the absence of hydrogen.
2 Experimental section
2.1 Materials
Crotonic acid (98 wt%) and polyphosphoric acid (115% H3PO4 basis) were purchased from Sigma-Aldrich (St Louis, MO, USA). Pre-determined concentrations (50 wt% to 100 wt%) of H3PO4 were prepared from polyphosphoric acid and distilled de-ionized water. Poly(3-hydroxybutyrate) (PHB 98 wt%) was obtained from Bio-on (Bologna, Italy). Its weight-average molecular weight (138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and number-average molecular weight (53
000 Da) and number-average molecular weight (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 Da) were measured with gel permeation chromatography (GPC) and calibrated with polystyrene standards. The polystyrene standards with narrow molecular weight distribution were purchased from Sigma-Aldrich.
100 Da) were measured with gel permeation chromatography (GPC) and calibrated with polystyrene standards. The polystyrene standards with narrow molecular weight distribution were purchased from Sigma-Aldrich.
2.2 Formation and analysis of hydrocarbon oil
In a typical experiment, 0.5 g PHB or crotonic acid and 10 mL of H3PO4 solution were put into a 20 mL polytetrafluoroethylene (PTFE) reactor, and the reactor was purged with N2 for about 10 minutes. The PTFE reactor was then sealed and left for a predetermined reaction time in a thermostat oven that was maintained at a desired temperature (165 to 240 °C). After reaction, the PTFE reactor was quickly cooled down in tap water. The reaction solution consisted of a top layer of oil products and a bottom layer of aqueous phosphoric acid solution. The oil was recovered by extraction with methylene chloride, and the water moisture of solvent solution was removed with anhydrous magnesium sulfate. After evaporation of methylene chloride at 40 °C, the oil was weighted to calculate the yield from the initial amount of PHB or crotonic acid, excluding the residual crotonic acid. During the evaporation of methylene chloride in the extracted oil samples, a control of pure methylene chloride with the same volume was put in the same condition at the same time. After the pure methylene chloride was completely evaporated, the extracted oil samples was kept in the evaporation condition for a little longer time (1–2 minutes) to make sure that the solvent methylene chloride was also completely evaporated. Duplicates or triplicates were performed to get an average yield and error range. In the experiment of phosphoric acid reuse, fresh PHB was added into the used H3PO4 solution to conduct the reaction under the same conditions as described above.
In order to determine the residual crotonic acid after reaction, the methylene chloride solution and the H3PO4 solution after solvent extraction were analyzed by using a gas chromatograph equipped with a flame ionization detector (GC-FID, Bruker 450-GC, CA, USA) and a high performance liquid chromatograph (HPLC, Shimadzu, Japan), respectively. The oil products in methylene chloride were analyzed with a gas chromatograph-mass spectrometer (GC-MS, Bruker 436-GC, CA, USA), a Fourier transform infrared spectrophotometer (FT-IR, Avatar 370, ThermoNicolet, FL, USA), and a carbon-13 nuclear magnetic resonance spectrometer (13C-NMR, Varian Unity Inova 400 MHz), respectively. Deuterated chloroform was used as the solvent in 13C-NMR analysis. The residual chemicals left in the H3PO4 solution before and after extraction were also analyzed with FT-IR, and phosphorus-31 nuclear magnetic resonance spectroscopy (31P-NMR, Varian Unity Inova 500 MHz), and a total organic carbon (TOC) analyzer. The TOC results were used for carbon recovery analysis along with the determination of major gas products as shown below.
2.3 Gas formation and analysis
In a typical experiment, 3.6 g of PHB or crotonic acid and 72 mL of H3PO4 solution were added into a 180 mL pyro-beaker, which was put in a 600 mL autoclave (Parr Instrument, IL, USA). The autoclave was then purged with nitrogen at least ten times of the reactor volume to remove air. The reactor was sealed and heated to a pre-determined temperature. When the temperature reached the setting value in about half an hour, the reaction time was set as zero, and thereafter recorded. After reaction, the reactor was cooled down in ambient conditions and gas samples were taken by using a FT-IR gas cell for qualitative analysis. The quantitative determination of CO2 and propylene was performed by using a gas chromatograph equipped with a thermal conductivity detector (GC-TCD, Bruker 450-GC, FL, USA) and a Carboxen-1006 Plot (30 m × 0.53 mm) column. Both CO2 and propylene were calibrated with pure gases against helium.
3 Results and discussion
3.1 Oil products analysis
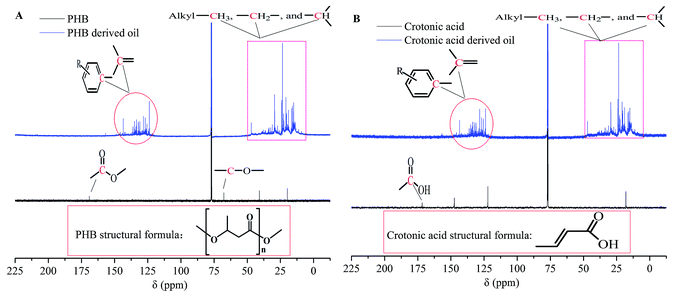
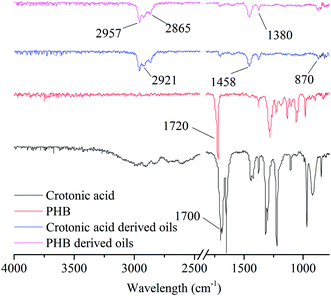
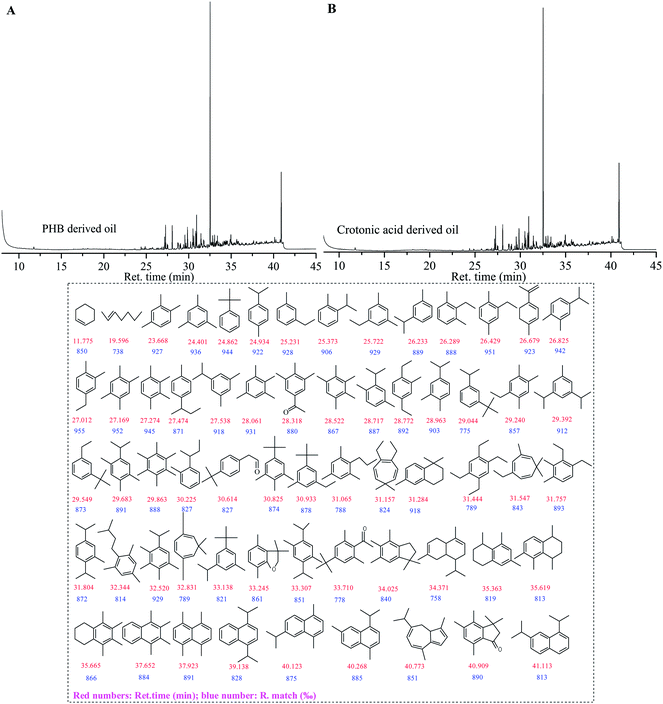
The PHB- and crotonic acid-derived oils produced in typical reaction conditions (100 wt% H3PO4, 220 °C, 3 hours) were analyzed by 13C-NMR (Fig. 1), FT-IR (Fig. 2), and GC-MS (Fig. 3), respectively. The analysis indicates that the oil products derived from both crotonic acid and PHB are almost the same. Comparing the 13C-NMR spectra of the raw materials (crotonic acid and PHB) with that of their oil products indicates that the carboxyl groups were almost completely removed (Fig. 1), and aromatic, alkene and alkane groups were formed in the oil products. This is confirmed with FTIR analysis (Fig. 2). The huge absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1700 cm−1 for crotonic acid and 1720 cm−1 for PHB disappeared in the oil products. The peaks (3100 to 2800 cm−1, 1458 cm−1, 1380 cm−1, and 870 cm−1) of oil products indicate the presence of methyl, methylene, and aromatic groups as the major groups. There is a very small peak around 1710 cm−1 in the FT-IR spectra of both crotonic acid and PHB derived oils, which may come from some aldehyde and/or ketone compounds. With the GC-MS analysis, a few aldehydes and ketones (e.g., retention time of 28.318 and 30.614, in Fig. 3) were detected. The GC-MS analysis also confirms that the carboxylic group of organic acid and ester were almost completely removed, and aromatics are the main products. These analytical results consistently indicate that PHB was to a great extent deoxygenated at a quite low temperature (220 °C) with formation of various hydrocarbons in one pot reaction in the absence of hydrogen. Compared with the conventional deoxygenation methods (e.g., pyrolysis and hydrotreating), this reaction system has some unique advantages in low reaction temperature and absence of hydrogen. Because of the same products formed from both PHB and crotonic acid, crotonic acid may be the key intermediate of PHB decarboxylation.
O at 1700 cm−1 for crotonic acid and 1720 cm−1 for PHB disappeared in the oil products. The peaks (3100 to 2800 cm−1, 1458 cm−1, 1380 cm−1, and 870 cm−1) of oil products indicate the presence of methyl, methylene, and aromatic groups as the major groups. There is a very small peak around 1710 cm−1 in the FT-IR spectra of both crotonic acid and PHB derived oils, which may come from some aldehyde and/or ketone compounds. With the GC-MS analysis, a few aldehydes and ketones (e.g., retention time of 28.318 and 30.614, in Fig. 3) were detected. The GC-MS analysis also confirms that the carboxylic group of organic acid and ester were almost completely removed, and aromatics are the main products. These analytical results consistently indicate that PHB was to a great extent deoxygenated at a quite low temperature (220 °C) with formation of various hydrocarbons in one pot reaction in the absence of hydrogen. Compared with the conventional deoxygenation methods (e.g., pyrolysis and hydrotreating), this reaction system has some unique advantages in low reaction temperature and absence of hydrogen. Because of the same products formed from both PHB and crotonic acid, crotonic acid may be the key intermediate of PHB decarboxylation.
 |
| | Fig. 1 13C-NMR spectra of PHB, crotonic acid and their oils produced in 100 wt% H3PO4 at 220 °C for 3 hours. | |
 |
| | Fig. 2 FT-IR spectra of PHB, crotonic acid and their oils produced in 100 wt% H3PO4 at 220 °C for 3 hours. | |
 |
| | Fig. 3 GC-MS analysis of PHB- and crotonic acid-derived oils produced in 100 wt% H3PO4 at 220 °C for 3 hours. | |
3.2 Gaseous products and analysis
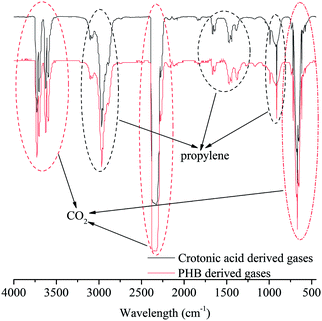
The gas products formed from PHB and crotonic acid deoxygenation were analyzed with FT-IR (Fig. 4). In comparison with FT-IR spectra of pure CO2, CO and propylene (Fig. S1, in ESI†), it was found that CO2 was the major gas product followed by propylene, with negligible amount of CO. In another words, PHB was deoxygenated primarily via decarboxylation (CO2), instead of decarbonylation (CO). Based on complete decarboxylation of a PHB monomer or crotonic acid (C4H6O2 → C3H6 + CO2), the theoretical yield of CO2 is 51.2 wt% of initial PHB. Actually, the detected CO2 in gas phase accounts for 35.3 wt%, or 68.9% of maximum yield. It implies that at least 68.9% of oxygen in PHB was removed by decarboxylation. Some of oxygen might be removed by formation of water as discussed later. Similarly for crotonic acid, 70.8% of oxygen was removed as CO2 via decarboxylation. Along with decarboxylation, propylene was the major gaseous hydrocarbon formed from PHB or crotonic acid. A small amount of alkyl gases might also be formed, as indicated by the peaks at 3100, 2790, 1670, 1450, 910 cm−1 in Fig. 4 and in comparison with pure propylene in Fig. S1 (in ESI†). Interestingly, the FT-IR analysis also indicates that crotonic acid and PHB have almost the same gas products (Fig. 4).
 |
| | Fig. 4 FT-IR spectra of PHB- and crotonic acid-derived gases. | |
3.3 Carbon recovery analysis
Since crotonic acid is the key intermediate of PHB decarboxylation and generates the very similar liquid and gas products, we used crotonic acid as the control reactant for carbon balance analysis of the reaction system. As shown in Table 1, the total carbon recovery from the initial carbon of crotonic acid was more than 90.9 wt%, which included CO2 (18.0 wt%) and propylene (4.9 wt%) in the gas phase, hydrocarbon oil (57.0 wt%) and organic carbon residues (11.0 wt%) in the liquid phase. Very little carbon (<2.1 wt%) was formed as char in solid phase. In addition to the loss of solid carbon, a small amount of carbon in the gas phase such as alkyl is also not included in the carbon balance, even though they were detected by FT-IR (Fig. 4). Compared with many biomass-to-liquid researches where only 25–45% of initial carbon was recovered,27 this research has a quite good carbon recovery. Importantly, the hydrocarbon oil contains 57.0 wt% of initial carbon, which is miscible with methylene chloride and hexane (data not shown here).
Table 1 Carbon recovery of crotonic acid decarboxylation and oil formationa
| Phase |
Products |
Carbon recoveryb (wt%) |
| The reaction was performed in 100 wt% H3PO4 solution at 220 °C for 3 hours. The carbon mass percentage recovered from the initial carbon of crotonic acid. The residual organic carbons left in H3PO4 solution. The carbon content of char is assumed 60 wt%. |
| Gas |
CO2 |
18.0 |
| C3H6 |
4.9 |
| Liquid |
Oil |
57.0 |
| Residues in H3PO4 solutionc |
11.0 |
| Solid |
Char |
<2.1%d |
| |
Total |
>90.9% |
3.4 Effect of H3PO4 concentration
Phosphoric acid is the catalyst in decarboxylation of PHB, and its concentration has a significant effect on PHB conversion and oil yield in typical reaction conditions (220 °C, 3 hours) as shown in Fig. 5. Crotonic acid was a major intermediate of PHB reaction in 50 wt% H3PO4. With increase of H3PO4 concentration, the residual crotonic acid content decreased, and little crotonic acid was remained in the 100 wt% H3PO4 and polyphosphoric acid (115% H3PO4 basis). The PHB-derived oil yield increased from 5.4 wt% to 37.3 wt% correspondingly with the increase of H3PO4 concentration from 50 wt% to 100 wt%. Little char (<2 wt%) was formed in these H3PO4 solutions. In polyphosphoric acid solution, however, a substantial amount of char (55.5 wt%) was formed, and the oil yield declined to 5.2 wt% of initial PHB. Carbonization of PHB or its derived hydrocarbons became the predominant reaction in polyphosphoric acid solution. A similar effect of phosphoric acid on crotonic acid was also observed and showed in Fig. 5. We suspect that PHB carbonization occurs after its thermal degradation into crotonic acid. This fact also supports our previous conclusion that crotonic acid is the key intermediate of PHB conversion into hydrocarbon oil.
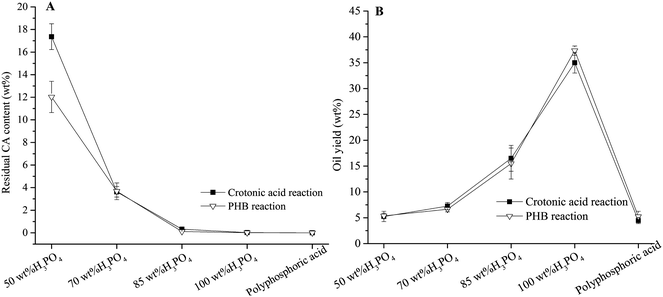 |
| | Fig. 5 The effect of H3PO4 concentration on residual crotonic acid content and oil yield at 220 °C for 3 hours. | |
The concentration of H3PO4 also affected the composition of the PHB-derived oils as revealed by their GC-MS chromatograms (Fig. 3 and S2 in ESI†). Table 2 gives the relative peak area (%) of the chemicals identified by GC-MS analysis. Specifically, aromatics (benzene and naphthalene derivatives) were the main oil products in 100 wt% H3PO4, and the naphthalene derivatives via ring condensation28 predominated in polyphosphoric acid solution. Formation of high C/H ratio hydrocarbons in high phosphoric acid is in agreement with our previous observation on char formation in polyphosphoric acid. A few unsaturated ketones and aldehydes were detected by GC-MS analysis, which may be produced together with CO2 and water by phosphoric acid catalytic deoxygenation of PHB/crotonic acid in considering of former reports.16,29,30 Furthermore, the existence of unsaturated ketones and aldehydes implies a possible way for aromatics formation, which may occur by some transient intermediate ketones and aldehydes through acid catalytic aldol condensation, dehydration and aromatization reactions.31–33 In diluted phosphoric acid solutions (70–85 wt%), however, acyclic alkenes and alkanes or cyclo-alkenes and alkanes became the important oil compounds, which means aromatization reactions should be somewhat inhibited. In 50 wt% H3PO4, the O-containing compounds were the main products, indicating that PHB deoxygenation was not completed yet. For conclusion, phosphoric acid concentration is a potential tool in controlling oil yield, deoxygen extent, and the hydrocarbon composition.
Table 2 Chemical distributiona of PHB-derived oils generated in different phosphoric acid solutions at 220 °C for 3 hours
| |
Relative peak area (%) |
| O-containing compounds |
Acyclic alkanes |
Cycloalkanes |
Acyclic alkenes |
Cycloalkenes |
Benzenes |
Naphthalenes |
| The chemical structures are identified with GC-MS. |
| 50 wt% H3PO4 |
66.3 |
3.7 |
1.6 |
16.7 |
3.9 |
7.7 |
0 |
| 70 wt% H3PO4 |
27.5 |
1.7 |
1.0 |
61.7 |
3.8 |
4.3 |
0 |
| 85 wt% H3PO4 |
16.2 |
3.2 |
3.7 |
57.1 |
1.7 |
18.1 |
0 |
| 100 wt% H3PO4 |
15.2 |
0.7 |
0.4 |
0.2 |
5.2 |
72.0 |
6.3 |
| Polyphosphoric acid |
0 |
0 |
0 |
0 |
18.4 |
52.6 |
28.9 |
Furthermore, high concentration of H3PO4 was favorable to formation of hydrocarbons with high carbon numbers (Table 3). C4–C9 compounds were the main products in 50 wt% H3PO4 solution while C10–C15 became the main products in 100 wt% H3PO4 solution. Based on the carbon numbers and the content of aromatics,34 the oil produced in 100 wt% H3PO4 could be a ‘drop in’ gasoline. According to the residual crotonic acid content, oil yield and product distribution, 100 wt% H3PO4 is an interesting catalyst solution for PHB deoxygenation. We further investigated the influence of reaction time and temperature on PHB conversion and oil yield.
Table 3 Carbon distributiona of PHB-derived oils formed in different phosphoric acid solutions at 220 °C for 3 hours
| |
Relative peak area (%) |
| C4 |
C5 |
C6 |
C7 |
C8 |
C9 |
C10 |
C11 |
C12 |
C13 |
C14 |
C15 |
C16 |
| The carbon numbers distributions are based GC-MS analysis. |
| 50 wt% H3PO4 |
12.1 |
4.6 |
5.3 |
31.2 |
5.9 |
24.3 |
2.6 |
0.6 |
4.6 |
8.8 |
0 |
0 |
0 |
| 70 wt% H3PO4 |
5.5 |
0 |
3.1 |
20 |
2.7 |
59.6 |
4.9 |
0 |
0.3 |
3.6 |
0.3 |
0 |
0 |
| 85 wt% H3PO4 |
0.8 |
0 |
0.7 |
4.5 |
5.1 |
34.2 |
21.2 |
6.8 |
8.8 |
13.6 |
1.8 |
2.5 |
0 |
| 100 wt% H3PO4 |
0 |
0 |
0.4 |
0 |
0.2 |
1.1 |
8.5 |
5.3 |
13.1 |
40.5 |
24.7 |
6.2 |
0 |
| Polyphosphoric acid |
0 |
0 |
18.4 |
0 |
0 |
0.2 |
13.6 |
8 |
10 |
20.9 |
6.2 |
18.0 |
4.7 |
3.5 Effect of reaction temperature and time
Temperature had apparent effect on residual crotonic acid content and oil yield from 165 to 220 °C in both PHB and crotonic acid reactions (Fig. 6). The residual crotonic acid content decreased while the oil yields increased with the increase of temperature. Little residual crotonic acid was detected at 220 °C or above, indicating that this mild temperature is high enough for complete conversion of PHB and crotonic acid. The highest oil yield (38.2 wt%) of PHB was obtained at 230 °C, and further increase of temperature didn't result in higher oil yield. This oil yield is very high, compared with the highest hydrocarbon oil yield (17.78 wt%) from catalytic co-deoxy-liquefaction of biomass and vegetable oil at 350–500 °C.35 Besides, the reaction temperature is also much lower than those of conventional biomass deoxygenation. Interestingly, the PHB derived oils produced at different temperatures have very similar peak distribution in their GC-MS chromatograms (Fig. S3, in ESI†). This fact indicates that temperature is not an important factor in determining the oil composition.
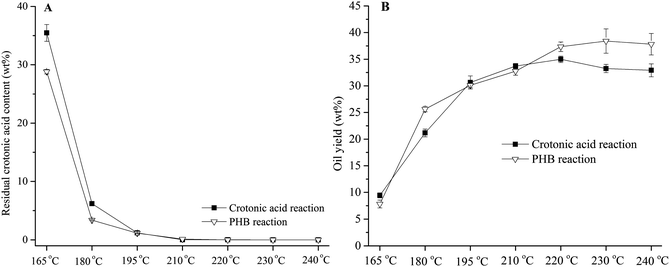 |
| | Fig. 6 The effect of reaction temperature on residual crotonic acid content and oil yield in 100 wt% H3PO4 for 3 hours. | |
The influence of reaction time on PHB conversion was tested in 100 wt% H3PO4 at 220 °C (Fig. 7). Three hours seemed sufficient for complete conversion of PHB, as little crotonic acid was detected in both PHB and crotonic acid reactions. Extension of reaction time (e.g., 6 or 9 hours) seems not beneficial, because the yield of PHB-derived oil declined to some extent with extended reaction time. Some products such as char which are not soluble in methylene chloride were formed in extended reaction. As mentioned in the method of oil recovery, the insoluble products are not accounted for hydrocarbon oil. On the other hand, the influence of reaction time on PHB conversion showed quite similar results of crotonic acid conversion, which implies again that crotonic acid is the key intermediate of hydrocarbon oil production.
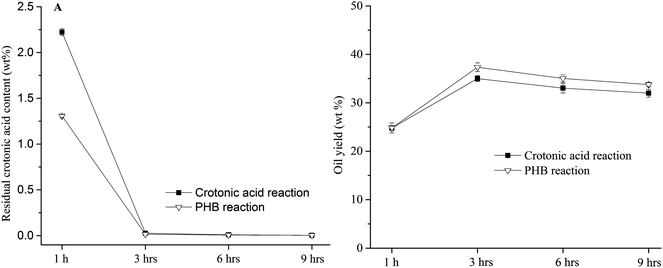 |
| | Fig. 7 The effect of reaction time on residual crotonic acid content and oil yield in 100 wt% at 220 °C. | |
3.6 Reuse of H3PO4 solution
As shown in Fig. 8, the PHB-derived oil yield in a 100 wt% H3PO4 was consistently higher than 32% in repeated use for 6 times. We compared the FT-IR spectra of the fresh and used acid solution (Fig. S4 in ESI†), and found no visible change. We also compared the P-NMR spectra of the acid solutions (Fig. S5, in ESI†) and found that H4P2O7 in fresh acid solution disappeared in the reused acid solutions. Most likely, A self-dissociation reaction of H4P2O7 occurred in the solution (2H3PO4 ⇆ H4P2O7 + H2O)25,36,37 and the water was provided from PHB deoxygenation. In another words, some oxygen was removed as H2O, forming high C/H hydrocarbons or even char. We also demonstrated that oil yield declined in diluted acid solutions. This may explain why the oil yield declines a little bit with water formation in the reused acid solution.
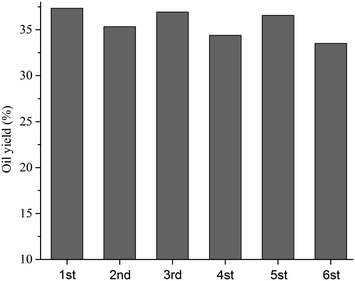 |
| | Fig. 8 The yield of PHB-derived hydrocarbon oil in a reused H3PO4 (100 wt%) solution at 220 °C for 3 hours. | |
3.7 Reaction mechanism
As shown above, crotonic acid is the key intermediate in conversion of PHB into hydrocarbon oil. Research on PHB thermal degradation has revealed that two neighboring monomeric units of a PHB backbone form a transient structure of a six-member ring, resulting in one unsaturated end and one carboxylic acid end.38,39 Sequential or simultaneous degradation of the oligomers finally generates crotonic acid. It is widely accepted that this transient structure occurs predominantly in thermal degradation of PHB, particularly at the melting point (around 180 °C) or above because the PHB backbone has a great flexibility to form the planar six-membered ring structure.
Decarboxylation of crotonic acid (C4H6O4 → CO2 + C3H6) occurs because of conjugation of the unsaturated bond (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and the carboxy bond (C
C–) and the carboxy bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The promotion effect of the conjugation effect is confirmed when butanoic acid, a saturated C4 acid, was treated in the same conditions, but generating little deoxygenated products (data not shown here). Obviously, propylene is formed along with decarboxylation of crotonic acid. We originally believed that propylene undergoes radical oligomerization to generate a complex mixture of hydrocarbons (>C6). However, when propylene was used as a gas reactant, the hydrocarbons formed were not identical with those formed from crotonic acid and PHB (Fig. 3). Specifically, we observed two major products derived from both crotonic acid and PHB and their retention times in GC-MS chromatograms are 32.520 min and 41.909 min, respectively. They are tentatively identified as a 1,2,3,4-tetramethyl-5-(1-methylethyl)-benzene and a 3,3,4,5,7-pentamethyl-1-indanone based on their high match probability (≥890‰) of MS spectra (Fig. 3). The lack of these two major products from propylene reaction implies that propylene is not a key intermediate but a byproduct of deoxygenation of crotonic acid and PHB. A very active intermediate might be formed in decarboxylation of crotonic acid, and hydrocarbons could be formed directly from the intermediate rather than from propylene. It should be pointed out that propylene can also be converted into hydrocarbon products with phosphoric acid catalysts.24,40–42 Further investigation is needed on the reaction mechanism, especially on what happens after crotonic acid is formed from PHB degradation.
O). The promotion effect of the conjugation effect is confirmed when butanoic acid, a saturated C4 acid, was treated in the same conditions, but generating little deoxygenated products (data not shown here). Obviously, propylene is formed along with decarboxylation of crotonic acid. We originally believed that propylene undergoes radical oligomerization to generate a complex mixture of hydrocarbons (>C6). However, when propylene was used as a gas reactant, the hydrocarbons formed were not identical with those formed from crotonic acid and PHB (Fig. 3). Specifically, we observed two major products derived from both crotonic acid and PHB and their retention times in GC-MS chromatograms are 32.520 min and 41.909 min, respectively. They are tentatively identified as a 1,2,3,4-tetramethyl-5-(1-methylethyl)-benzene and a 3,3,4,5,7-pentamethyl-1-indanone based on their high match probability (≥890‰) of MS spectra (Fig. 3). The lack of these two major products from propylene reaction implies that propylene is not a key intermediate but a byproduct of deoxygenation of crotonic acid and PHB. A very active intermediate might be formed in decarboxylation of crotonic acid, and hydrocarbons could be formed directly from the intermediate rather than from propylene. It should be pointed out that propylene can also be converted into hydrocarbon products with phosphoric acid catalysts.24,40–42 Further investigation is needed on the reaction mechanism, especially on what happens after crotonic acid is formed from PHB degradation.
4 Conclusions
PHB is a type of new renewable feedstock from which hydrocarbon oil is effectively produced in a simple one-pot reaction. Compared to conventional biomass processing, PHB processing demonstrated in this work has some technical advantages such as mild reaction temperature (around 220 °C), cheap reusable catalyst (80–100 wt% H3PO4), and high oil yield (38.2 wt%) without using hydrogen.
Acknowledgements
The work is supported by the Office of Naval Research, USA (Award N00014-13-1-0463).
Notes and references
- S. Khanna and A. K. Srivastava, Process Biochem., 2005, 40, 607–619 CrossRef CAS PubMed.
- N. Tanadchangsaeng and J. Yu, Biotechnol. Bioeng., 2012, 109, 2808–2818 CrossRef CAS PubMed.
- G. Du, J. Chen, J. Yu and S. Lun, J. Biotechnol., 2001, 88, 59–65 CrossRef CAS.
- R. Nonato, P. Mantelatto and C. Rossell, Appl. Microbiol. Biotechnol., 2001, 57, 1–5 CrossRef CAS.
- M. Akiyama, T. Tsuge and Y. Doi, Polym. Degrad. Stab., 2003, 80, 183–194 CrossRef CAS.
- S. Godbole, S. Gote, M. Latkar and T. Chakrabarti, Bioresour. Technol., 2003, 86, 33–37 CrossRef CAS.
- J. Yu and L. X. L. Chen, Biotechnol. Prog., 2006, 22, 547–553 CrossRef CAS PubMed.
- J. Yu, Trends Biotechnol., 2013, 32, 5–10 CrossRef PubMed.
- A. Gonzalez, L. Irusta, M. J. Fernández-Berridi, M. Iriarte and J. J. Iruin, Polym. Degrad. Stab., 2005, 87, 347–354 CrossRef CAS PubMed.
- J. Yu, D. Plackett and L. X. Chen, Polym. Degrad. Stab., 2005, 89, 289–299 CrossRef CAS PubMed.
- C. R. Fischer, A. A. Peterson and J. W. Tester, Ind. Eng. Chem. Res., 2011, 50, 4420–4424 CrossRef CAS.
- D. Wang, S. H. Hakim, D. M. Alonso and J. A. Dumesic, Chem. Commun., 2013, 49, 7040–7042 RSC.
- F. de M. Mercader, M. J. Groeneveld, S. R. A. Kersten, C. Geantet, G. Toussaint, N. W. J. Way, C. J. Schaverien and K. J. A. Hogendoorn, Energy Environ. Sci., 2011, 4, 985 CAS.
- S. J. Eduardo and M. Crocker, J. Chem. Technol. Biotechnol., 2012, 87, 1041–1050 CrossRef.
- A. G. Gayubo, A. T. Aguayo, A. Atutxa, R. Aguado, M. Olazar and J. Bilbao, Ind. Eng. Chem. Res., 2004, 43, 2619–2626 CrossRef CAS.
- J. D. Adjaye and N. N. Bakhshi, Biomass Bioenergy, 1995, 8, 131–149 CrossRef CAS.
- M. Bertero, G. de la Puente and U. Sedran, Renewable Energy, 2013, 60, 349–354 CrossRef CAS PubMed.
- T. J. Schwartz, A. R. P. van Heiningen and M. C. Wheeler, Green Chem., 2010, 12, 1353–1356 RSC.
- N. Taufiqurrahmi and S. Bhatia, Energy Environ. Sci., 2011, 4, 1087–1112 CAS.
- M. A. Abdelwahab, A. Flynn, B. Chiou, S. Imam, W. Orts and E. Chiellini, Polym. Degrad. Stab., 2012, 97, 1822–1828 CrossRef CAS PubMed.
- S. N. lee, M. Y. Lee and W. H. Park, J. Appl. Polym. Sci., 2002, 83, 2945–2952 CrossRef CAS.
- C. S. Ki, K. H. Lee, D. H. Baek, M. Hattori, I. C. Um, D. W. Ihm and Y. H. Park, J. Appl. Polym. Sci., 2007, 105, 1605–1610 CrossRef CAS.
- H. Boerstoel, H. Maatman, J. B. Westerink and B. M. Koenders, Polymer, 2001, 42, 7371–7379 CrossRef CAS.
- S. R. Bethea and J. H. Karchmer, Ind. Eng. Chem., 1956, 48, 370–377 CrossRef CAS.
- A. de Klerk, D. O. Leckel and N. M. Prinsloo, Ind. Eng. Chem. Res., 2006, 45, 6127–6136 CrossRef CAS.
- R. Bekker and N. M. Prinsloo, Ind. Eng. Chem. Res., 2009, 48, 10156–10162 CrossRef CAS.
- D. Unruh, K. Pabst and G. Schaub, Energy Fuels, 2010, 24, 2634–2641 CrossRef CAS.
- C. K. Bradsher, Chem. Rev., 1987, 87, 1277–1297 CrossRef CAS.
- A. G. Gayubo, A. T. Aguayo, A. Atutxa, R. Aguado, M. Olazar and J. Bilbao, Ind. Eng. Chem. Res., 2004, 43, 2619–2626 CrossRef CAS.
- M. A. Alotaibi, E. F. Kozhevnikova and I. V. Kozhevnikov, Appl. Catal., A, 2012, 447–448, 32–40 CrossRef CAS PubMed.
- A. de Klerk, R. J. J. Nel and R. Schwarzer, Ind. Eng. Chem. Res., 2007, 46, 2377–2382 CrossRef CAS.
- M. Bertero, G. de la Puente and U. Sedran, Renewable Energy, 2013, 60, 349–354 CrossRef CAS PubMed.
- T. Xu, E. J. Munson and J. F. Haw, J. Am. Chem. Soc., 1994, 116, 1962–1972 CrossRef CAS.
- W. J. Pitz and C. J. Mueller, Prog. Energy Combust. Sci., 2011, 37, 330–350 CrossRef CAS PubMed.
- Y. Chen, F. Yang, L. Wu, C. Wang and Z. Yang, Bioresour. Technol., 2011, 102, 1933–1941 CrossRef CAS PubMed.
- W. H. Ross and R. M. Jones, J. Am. Chem. Soc., 1925, 47, 2165–2170 CrossRef CAS.
- R. A. Munson, J. Phys. Chem., 1964, 68, 3374–3377 CrossRef CAS.
- H. Ariffin, H. Nishida, Y. Shirai and M. A. Hassan, Polym. Degrad. Stab., 2008, 93, 1433–1439 CrossRef CAS PubMed.
- H. Nishida, H. Ariffin, Y. Shirai, and M. A. Hassan, in Biopolymers, ed. M. Elnashar, Sciyo, Rijeka, 2010, vol. 19, pp. 369–386 Search PubMed.
- Z. Zhu, Z. Xie, Y. Chen, R. F. Wang and Y. P. Yao, React. Kinet. Catal. Lett., 2000, 70, 379–388 CrossRef CAS.
- T. M. Sakuneka, A. de Klerk, R. J. J. Nel and A. D. Pienaar, Ind. Eng. Chem. Res., 2008, 47, 1828–1834 CrossRef CAS.
- T. F. Degnan, C. M. Smith and C. R. Venkat, Appl. Catal., A, 2001, 221, 283–294 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR spectra of CO2, CO, propylene, and various status of H3PO4 solution; GC-MS analysis of PHB derived oils produced at various H3PO4 concentrations and temperatures; 31P-NMR analysis of fresh 100 wt% H3PO4 and the H3PO4 solution after reaction. See DOI: 10.1039/c4ra00892h |
|
| This journal is © The Royal Society of Chemistry 2014 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) and number-average molecular weight (53
000 Da) and number-average molecular weight (53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 Da) were measured with gel permeation chromatography (GPC) and calibrated with polystyrene standards. The polystyrene standards with narrow molecular weight distribution were purchased from Sigma-Aldrich.
100 Da) were measured with gel permeation chromatography (GPC) and calibrated with polystyrene standards. The polystyrene standards with narrow molecular weight distribution were purchased from Sigma-Aldrich.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1700 cm−1 for crotonic acid and 1720 cm−1 for PHB disappeared in the oil products. The peaks (3100 to 2800 cm−1, 1458 cm−1, 1380 cm−1, and 870 cm−1) of oil products indicate the presence of methyl, methylene, and aromatic groups as the major groups. There is a very small peak around 1710 cm−1 in the FT-IR spectra of both crotonic acid and PHB derived oils, which may come from some aldehyde and/or ketone compounds. With the GC-MS analysis, a few aldehydes and ketones (e.g., retention time of 28.318 and 30.614, in Fig. 3) were detected. The GC-MS analysis also confirms that the carboxylic group of organic acid and ester were almost completely removed, and aromatics are the main products. These analytical results consistently indicate that PHB was to a great extent deoxygenated at a quite low temperature (220 °C) with formation of various hydrocarbons in one pot reaction in the absence of hydrogen. Compared with the conventional deoxygenation methods (e.g., pyrolysis and hydrotreating), this reaction system has some unique advantages in low reaction temperature and absence of hydrogen. Because of the same products formed from both PHB and crotonic acid, crotonic acid may be the key intermediate of PHB decarboxylation.
O at 1700 cm−1 for crotonic acid and 1720 cm−1 for PHB disappeared in the oil products. The peaks (3100 to 2800 cm−1, 1458 cm−1, 1380 cm−1, and 870 cm−1) of oil products indicate the presence of methyl, methylene, and aromatic groups as the major groups. There is a very small peak around 1710 cm−1 in the FT-IR spectra of both crotonic acid and PHB derived oils, which may come from some aldehyde and/or ketone compounds. With the GC-MS analysis, a few aldehydes and ketones (e.g., retention time of 28.318 and 30.614, in Fig. 3) were detected. The GC-MS analysis also confirms that the carboxylic group of organic acid and ester were almost completely removed, and aromatics are the main products. These analytical results consistently indicate that PHB was to a great extent deoxygenated at a quite low temperature (220 °C) with formation of various hydrocarbons in one pot reaction in the absence of hydrogen. Compared with the conventional deoxygenation methods (e.g., pyrolysis and hydrotreating), this reaction system has some unique advantages in low reaction temperature and absence of hydrogen. Because of the same products formed from both PHB and crotonic acid, crotonic acid may be the key intermediate of PHB decarboxylation.







![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–) and the carboxy bond (C
C–) and the carboxy bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). The promotion effect of the conjugation effect is confirmed when butanoic acid, a saturated C4 acid, was treated in the same conditions, but generating little deoxygenated products (data not shown here). Obviously, propylene is formed along with decarboxylation of crotonic acid. We originally believed that propylene undergoes radical oligomerization to generate a complex mixture of hydrocarbons (>C6). However, when propylene was used as a gas reactant, the hydrocarbons formed were not identical with those formed from crotonic acid and PHB (Fig. 3). Specifically, we observed two major products derived from both crotonic acid and PHB and their retention times in GC-MS chromatograms are 32.520 min and 41.909 min, respectively. They are tentatively identified as a 1,2,3,4-tetramethyl-5-(1-methylethyl)-benzene and a 3,3,4,5,7-pentamethyl-1-indanone based on their high match probability (≥890‰) of MS spectra (Fig. 3). The lack of these two major products from propylene reaction implies that propylene is not a key intermediate but a byproduct of deoxygenation of crotonic acid and PHB. A very active intermediate might be formed in decarboxylation of crotonic acid, and hydrocarbons could be formed directly from the intermediate rather than from propylene. It should be pointed out that propylene can also be converted into hydrocarbon products with phosphoric acid catalysts.24,40–42 Further investigation is needed on the reaction mechanism, especially on what happens after crotonic acid is formed from PHB degradation.
O). The promotion effect of the conjugation effect is confirmed when butanoic acid, a saturated C4 acid, was treated in the same conditions, but generating little deoxygenated products (data not shown here). Obviously, propylene is formed along with decarboxylation of crotonic acid. We originally believed that propylene undergoes radical oligomerization to generate a complex mixture of hydrocarbons (>C6). However, when propylene was used as a gas reactant, the hydrocarbons formed were not identical with those formed from crotonic acid and PHB (Fig. 3). Specifically, we observed two major products derived from both crotonic acid and PHB and their retention times in GC-MS chromatograms are 32.520 min and 41.909 min, respectively. They are tentatively identified as a 1,2,3,4-tetramethyl-5-(1-methylethyl)-benzene and a 3,3,4,5,7-pentamethyl-1-indanone based on their high match probability (≥890‰) of MS spectra (Fig. 3). The lack of these two major products from propylene reaction implies that propylene is not a key intermediate but a byproduct of deoxygenation of crotonic acid and PHB. A very active intermediate might be formed in decarboxylation of crotonic acid, and hydrocarbons could be formed directly from the intermediate rather than from propylene. It should be pointed out that propylene can also be converted into hydrocarbon products with phosphoric acid catalysts.24,40–42 Further investigation is needed on the reaction mechanism, especially on what happens after crotonic acid is formed from PHB degradation.
