A water-soluble highly sensitive and selective fluorescent sensor for Hg2+ based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole via ligand-to-metal charge transfer (LMCT)†
Abstract
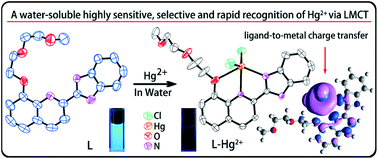
A water-soluble fluorescent sensor (L) based on 2-(2-(8-hydroxyquinolin)-yl)benzimidazole has been synthesized and characterized. Sensor L displays highly selective and sensitive recognition of Hg2+ with a fluorescence “ON–OFF” response in buffered aqueous solution (1% dimethyl sulfoxide (DMSO), Tris–HCl 10 mM, pH = 7.4). X-ray crystal structure of the L–Hg2+ complex reveals that the oxygen and nitrogen atoms of 8-hydroxyquinoline and the imine N atom of the benzimidazole unit (N1) bind Hg2+ through a 1 : 1 binding stoichiometry. The fluorescence quenching mechanism is qualitatively evaluated by quantum chemical calculations which show that the fluorescence quenching phenomenon is caused by ligand-to-metal charge transfer (LMCT) in the excited state.

 Please wait while we load your content...
Please wait while we load your content...