Synthesis of nucleobase-caged peptide nucleic acids having improved photochemical properties†
Abstract
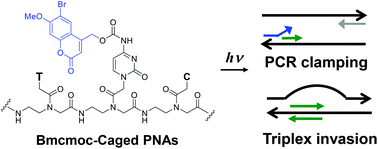
A nucleobase-caged peptide nucleic acid (PNA) having a (6-bromo-7-methoxycoumarin)-4-ylmethoxycarbonyl (Bmcmoc) caging group was newly synthesized. The Bmcmoc-caged PNAs were photolyzed to produce parent PNAs with a high photochemical efficiency. Introduction of a single Bmcmoc group was sufficient to suppress polymerase chain reaction (PCR) clamping activity and triplex invasion complex formation. Photo-mediated restoration of the PCR clamping activity was also demonstrated.

 Please wait while we load your content...
Please wait while we load your content...