Open Access Article
Open Access ArticleSulfonic acid functionalized hyperbranched poly(ether sulfone) as a solid acid catalyst
Yuta
Nabae
*,
Jie
Liang
,
Xuhui
Huang
,
Teruaki
Hayakawa
and
Masa-aki
Kakimoto
Department of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1 S8-26 Ookayama, Meguro-ku, Tokyo 152-8552, Japan. E-mail: nabae.y.aa@m.titech.ac.jp; Fax: +81 3 5734 2433; Tel: +81 3 5734 2429
First published on 19th May 2014
Abstract
Sulfonic acid functionalized hyperbranched poly(ether sulfone) (SHBPES) was studied as a novel type of solid acid catalyst. Various molecular weights of SHBPESs were tested for the esterification reaction between acetic acid and 1-butanol. The SO3H terminal groups of the SHBPESs work as catalytically active sites, but all tested SHBPESs are totally or partially soluble under the current reaction conditions. To overcome the solubility problem, SHBPES was grafted onto carbon black, and this material, SHBPES/CB, shows fairly good catalytic activity and promising recyclability. The turnover frequency of SHBPES decreased upon grafting it onto carbon black, but it was still much better than that of Amberlyst®-15. SHBPES/CB was also tested for the Friedel–Crafts alkylation of anisole and its durability seems to be much better than that of Amberlyst®-15 under the operating conditions at 130 °C.
Introduction
Acid catalyzed chemical reactions are widely used currently to produce various chemicals. In terms of green chemistry, it is quite important to replace liquid acid catalysts such as HF and H2SO4 with solid acid catalysts.1–3 Ion exchange polymers such as Amberlyst®-15 are typical solid acid catalysts; however, they are available only at ambient operating temperature, typically below 120 °C, because of low thermal stability of the vinyl based polymers.4,5 Developing ion exchange polymers with higher thermal stability will largely expand the applicability of polymer-based solid acid catalysts in various chemical processes.To develop a stable polymer-based solid acid catalyst, employing aromatic polymers such as poly(arylene ether sulfone) would be a reasonable approach because they are chemically and thermally stable.6,7 Besides, to develop a highly active catalyst material from such aromatic polymers, our research group is now interested in the hyperbranched structures. Hyperbranched polymers have a large number of end-groups and a dendritic-like structure.8–10 Unlike dendrimers, hyperbranched polymers can be synthesized in a one step process from ABx-type monomers, where x is two or more. It means that a large number of catalytically active sites can be introduced if the end-groups are converted into catalytically active functional groups. Other properties of hyperbranched polymers might also contribute to the catalytic activity of the end-groups. The end-groups of hyperbranched polymers would be well exposed and accessible for the reactants of catalytic reactions because of a low degree of entanglement. Besides, a large free volume of hyperbranched polymers would enhance mass transport of the reactants and products. In the case of solid acid catalysts, a sulfonic acid functionalized hyperbranched polymer could be a good catalyst material.
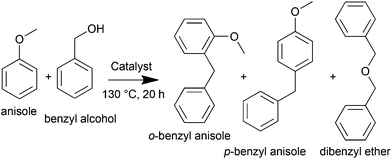
In this context, we hereby propose sulfonic acid functionalized hyperbranched poly(ether sulfone) (SHBPES)11,12 as a novel material for solid acid catalysts. This paper discusses the catalytic performance of SHBPESs for (1) the esterification between acetic acid and 1-butanol and (2) the Friedel–Crafts alkylation of anisole with benzyl alcohol. In addition, since SHBPES itself has a solubility problem as a solid catalyst, SHBPES immobilized onto carbon black (SHBPES/CB) has also been studied.
Experimental
Material synthesis
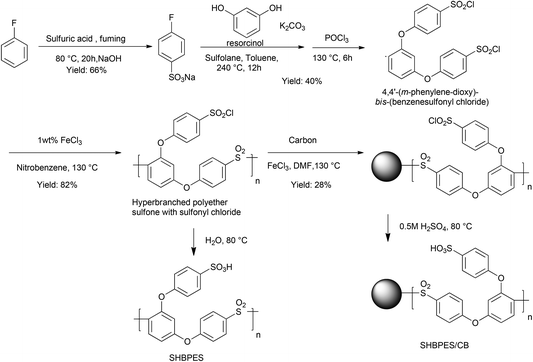
Scheme 1 shows the synthetic routes of SHBPES and SHBPES/CB. All reagents were used as received. Preparation of the AB2 monomer, 4,4′-(m-phenylene-dioxy)-bis-(benzenesulfonyl chloride), has been previously reported and the procedure was used with a slight modification.11,12 The prepared AB2 monomer (0.30 g, 0.65 mmol) was dissolved in nitrobenzene (1 mL) with FeCl3 (0.003 g, 0.018 mmol) and heated under N2 at 130 °C for 3–20 h. The mixture was poured into methanol with a small amount of HCl. The precipitate was washed with methanol, and then dried in vacuo at 80 °C. SHBPES was obtained by hydrolyzing the chloride form polymer in water at 80 °C for 20 h. To immobilize the polymer onto a carbon black, the chloride form polymer (0.2 g) was dissolved in N,N-dimethylformamide (DMF) with FeCl3 (0.01 g) and Ketjen Black (EC600JD, 0.1 g) and heated under N2 at 130 °C for 60 h. The resulting powder was stirred in DMF for two nights to remove unimmobilized polymer. SHBPES/CB was obtained by hydrolyzing the polymer/carbon sample in 0.5 M H2SO4 at 80 °C for 20 h.To elucidate the effect of the chemical structure of the backbone, the catalytic performances of p-toluenesulfonic acid (PTSA) and 4-(phenoxy)benzenesulfonic acid (PBSA) were also studied. PTSA was purchased in a monohydrate form (TCI) and used as obtained. PBSA was prepared by hydrolyzing 4-(phenoxy)benzenesulfonyl chloride, which was synthesized by the literature method.12
Measurements
Gel permeation chromatography (GPC) was performed for the chloride form polymer using a Viscotek GPC-1000 system equipped with a TDA 302 triple detector and a TSK-GEL α-M column. DMF with 0.05 M LiBr was used as an eluent. The weight average molecular weight (Mw) was calculated based on the light scattering data.The ion exchange capacity (IEC) of the prepared samples was determined by neutralization titration. The equivalent point was determined based on the primary differential of the pH-titration curve. For SHBPESs, a polymer powder (20 mg) was treated in a 5 M NaCl solution (10 ml) at room temperature and stirred overnight to exchange the protons on the sulfonic acid groups. Then the mixture was titrated with 0.013 M NaOH. For SHBPES/CB samples, a sample powder (50 mg) was treated in 0.1 M NaOH (3 ml) solution at room temperature and stirred overnight. The mixture was filtered and the remaining filtrate was titrated with 0.1 M HCl.
CHS elemental analysis was performed using a Perkinelmer 2400-II analyzer.
Transmission electron microscopy (TEM) was performed using a Hitachi H-7650 microscope operated at 100 kV. The sample was stained using a Gd based stainer (EM stainer, Nisshin EM) before the measurement.
Catalytic reaction
The catalytic performance of the materials was tested using a Shibata Chemist Plaza CP100 multi-reactor equipped with 100 mL reactors. The esterification over the prepared catalyst was performed by stirring acetic acid (20 mmol), 1-butanol (20 mmol) and the catalyst (20 mg) at 65 °C for 0.5–2.5 h. The Friedel–Crafts alkylation over the prepared catalyst was performed by stirring anisole (18.5 mmol), benzyl alcohol (1.25 mmol) and the catalyst (75 mg) at 130 °C for 20 h. The reaction products were quantified with a Shimadzu GCMS-QP2010 Plus equipped with a TC-FFAP column (0.25 mm ø × 30 m). The conversion was calculated based on the peak area ratio of 1-butanol or benzyl alcohol against the internal standard: naphthalene (esterification) or dodecane (Friedel–Crafts alkylation). The product yields were calculated based on the peak area ratios of the reaction products against the internal standard.Catalyst recycling tests were performed in the following manner. The reaction mixture was filtered after each run of the catalytic reaction, and then the residue was used for the next run after drying and weighing.
Results and discussion
Preparation of SHBPES
SHBPESs with various molecular weights were prepared by polymerizing the AB2 monomer, 4,4′-(m-phenylene-dioxy)-bis-(benzenesulfonyl chloride), for 3–20 h.11Table 1 shows Mw and IEC of the SHBPESs prepared in different reaction periods. The results suggest that the molecular weight of SHBPES was successfully controlled in the range of 3.6 × 104 to 2.06 × 105 g mol−1. SHBPES1 shows the highest IEC, 2.2 mmol g−1, whilst the theoretical value is 2.8 mmol g−1; and the IEC slightly decreases as the molecular weight increases. This is probably due to a cross-linking reaction between the SO2Cl terminals and aromatic rings during the polymerization.Catalytic performance of SHBPES
The SHBPESs were tested as a catalyst for the esterification reaction between acetic acid and 1-butanol. Fig. 1 shows the results of the esterification reaction with the SHBPESs. The results with Amberlyst®-15 and H2SO4 are also shown for comparison. H2SO4 showed the highest catalytic activity since this is a homogeneous catalyst. Amberlyst®-15, SHBPES4 and SHBPES5 showed similar catalytic performances, whilst SHBPES1, SHBPES2 and SHBPES3 showed better catalytic performances than Amberlyst®-15.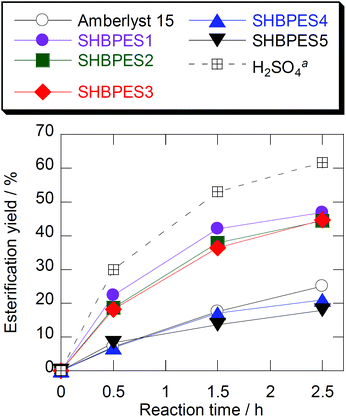 | ||
| Fig. 1 Time course of the esterification yield with various catalysts. Conditions: T 65 °C, acetic acid 0.02 mol, 1-butanol 0.02 mol, catalyst 20 mg. aH2SO4 equivalent to Amberlyst®-15 was used. | ||
Fig. 2 shows the results of recycling tests. After the first runs, most of SHBPES1, SHBPES2 and SHBPES3 could not be collected because the majority of polymers dissolved in the liquid phase. This is the reason why these SHBPESs showed better catalytic performances than Amberlyst®-15. These SHBPESs work as homogeneous catalysts under the current reaction conditions. The SHBPESs with higher molecular weights, SHBPES4 and SHBPES5, showed better collectability, but the recycled weight gradually decreased as the run number increased, whereas the recycle weight of Amberlyst®-15 was almost 100%. These experimental results suggest that the sulfonic acid terminals on SHBPESs can work as a solid catalyst, but the SHBPESs themselves are not suitable for a solid acid catalyst because of the solubility problem.
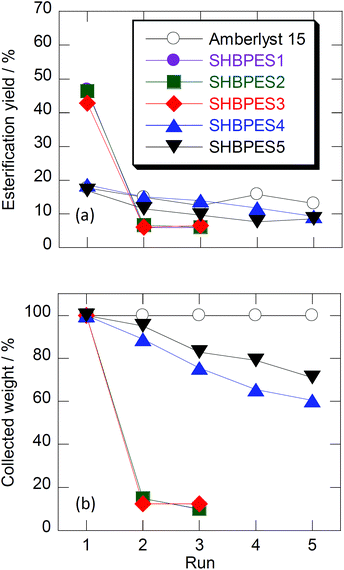 | ||
| Fig. 2 (a) Esterification yields with the fresh catalyst (1st run) and the recycle catalysts (2–5th run), and (b) the relative amounts of used catalyst which were collected from the previous run. Conditions: idem as Fig. 1 but the reaction period was 2.5 h. | ||
Immobilization of SHBPES onto carbon black
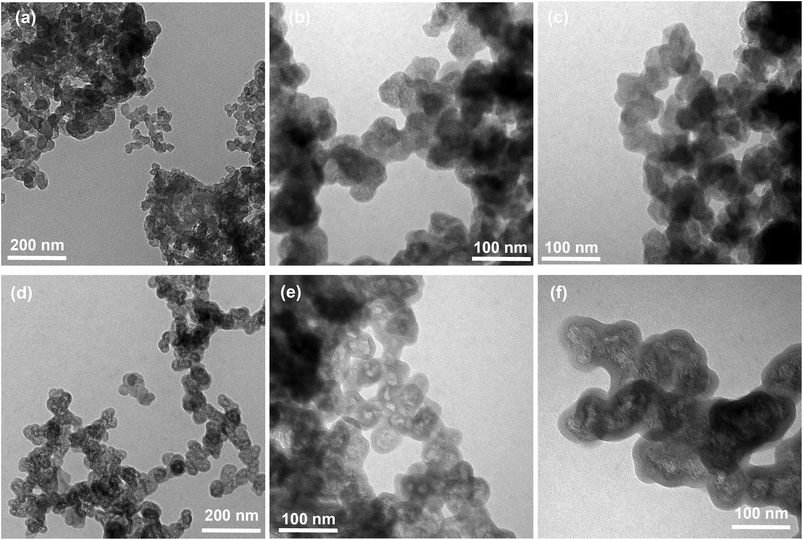
Since the SHBPESs themselves have a solubility problem to consider them as solid acid catalysts, we hereby discuss the immobilization of SHBPES onto a carbon black. Numerous reports have been published about grafting polymers onto carbon surfaces.13–21 Many of them employ hydrolyzable bonds such as ester and amide to graft polymers. To develop a reliable solid acid catalyst, however, such hydrolyzable bonds are not suitable because the materials will be used under strongly acidic conditions. In contrast, the present material, SHBPES, can be directly grafted onto carbon surfaces via the Friedel–Crafts reaction since the polymer can afford numerous sulfonyl chloride terminals.22 This will result in an aromatic polymer based solid acid catalyst without any hydrolyzable bond, and quite high durability can be expected under acidic conditions.Table 2 summarizes the results of immobilization of SHBPESs onto a carbon black via the Friedel–Crafts reaction as illustrated in Scheme 1. SHBPES1, SHBPES2 and SHBPES3 were selected for the immobilization considering the affinity between the polymer and the reaction phase for catalytic reactions. The IECs became 0.73–0.89 mmol g−1, whereas they were 2.08–2.20 mmol g−1 before the immobilization. These IECs correspond to the polymer loading of 31.7–38.7%. The polymer loading was also evaluated by CHS elemental analysis and the results are in good agreement with the values from the IECs. Although the polymer loading was not quite different among three samples, SHBPES3/CB showed the highest polymer loading. Thus obtained SHBPES3/CB was analyzed by TEM. Fig. 3 shows TEM images of SHBPES3/CB and pristine carbon black. Compared to pristine carbon black, the particle size of SHBPES3/CB seems slightly large. In the enlarged images, SHBPES3/CB seems to have a uniform polymer layer with a thickness of 10–20 nm. These experimental results suggest that SHBPES can be successfully grafted onto carbon black by the Friedel–Crafts reaction.
| Catalyst | IEC/mmol g−1 | Elemental analysis/wt% | Polymer loading/wt% | |||
|---|---|---|---|---|---|---|
| C | H | S | IECa | EAb | ||
| a Evaluated from the change in IEC before and after the immobilization. b Calculated from the S content of elemental analysis. | ||||||
| SHBPES1/CB | 0.87 | 77.3 | 2.2 | 5.1 | 38 | 35 |
| SHBPES2/CB | 0.73 | 76.7 | 2.1 | 5.1 | 32 | 35 |
| SHBPES3/CB | 0.89 | 75.9 | 2.3 | 5.3 | 39 | 36 |
SHBPES3/CB was studied as a solid acid catalyst for the esterification between acetic acid and 1-butanol. Table 3 compares the IEC and catalytic performance of several catalysts. The IEC of SHBPES3/CB was 0.89 mmol g−1 whereas that of SHBPES3 was 2.1 mmol g−1. SHBPES3/CB shows a fairly good catalytic activity: 13% of esterification yield against 46% by SHBPES3. The turnover frequency (TOF) of SHBPES3/CB is smaller than that of SHBPES3, but still better than that of Amberlyst®-15. The higher TOF against Amberlyst®-15 is probably due to the flexibility of the SO3H terminals on HBPES. The catalytic performance of SHBPES3/CB against other state-of-the-art catalysts could be supposed based on the comparison against that of Amberlyst®-15. Liu et al. compared various catalysts for the esterification between acetic acid and 1-butanol at 90 °C, and reported that H-PDVB-1.5-SO3H (IEC: 1.86 mmol g−1), SBA-15-SO3H (1.26 mmol g−1), H-beta zeolite (1.21 mmol g−1) and H-USY zeolite (2.06 mmol g−1) showed 9.3, 7.6, 6.7 and 4.1 min−1 of TOFs whereas Amberlyst®-15 showed 3.0 min−1.23 Considering the TOF of SHBPES3/CB in Table 3, which is three times better than that of Amberlyst®-15, it can be presumed that the inherent catalytic activity of the acidic terminal groups on HBPES is as good as the active sites of the state-of-the-art catalysts, although the mass catalytic activity is not because of the lower IEC.
| Catalyst | IEC/mmol g−1 | Catalyst amount/mg | Reaction period/h | Conversion/% | Yield/% | TOFb/min−1 |
|---|---|---|---|---|---|---|
| a Reaction conditions: idem as Fig. 1 but the reaction period and catalyst amount. b Calculated from the IEC and the esterification yield. c p-Toluene sulfonic acid. d 4-(Phenoxy)benzenesulfonic acid. | ||||||
| Blank | — | 2.5 | 3 | 4 | — | |
| Ketjen black | — | 20 | 2.5 | 4 | 4 | — |
| PTSAc | 5.3 | 10 | 0.5 | 33 | 26 | 3.21 |
| PBSAd | 4.0 | 10 | 0.5 | 26 | 21 | 3.56 |
| SHBPES3 | 2.1 | 20 | 0.5 | 20 | 18 | 2.85 |
| SHBPES3/CB | 0.89 | 20 | 2.5 | 13 | 13 | 0.97 |
| Amberlyst®-15 | 4.7 | 20 | 2.5 | 20 | 21 | 0.30 |
One might wonder whether the chemical structure of the polymer backbone significantly affects the catalytic activity of the terminal functional groups. To elucidate the effect of the backbone, the homogeneous catalysis of model compounds was studied. Table 3 shows the catalytic performances of PTSA and PBSA for the esterification between acetic acid and 1-butanol. PBSA showed a slightly better TOF but the catalytic activities of these two compounds were not very different. Therefore, the better TOF of SHBPES3/CB compared to that of Amberlyst®-15 presumably derives from good flexibility of the terminals on the hyperbranched structure rather than their chemical structures.
Recycling of SHBEPS3/CB was tested in the same manner as the experiments for SHBPESs and the results are shown in Fig. 4. Although the collecting yield of SHBPES3/CB is not 100%, SHBPES3/CB over 90% was successfully collected even after 5 runs. In the meantime, the catalytic activity of SHBPES3/CB seems fairly stable. It could be concluded that the stability of SHBPES3/CB is quite promising.
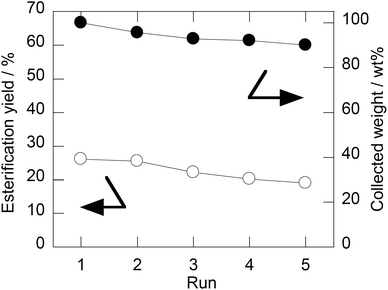 | ||
| Fig. 4 Esterification yields with the fresh catalyst (1st run) and the recycle catalysts (2–5th run), and the relative amounts of catalyst against the 1st run. Conditions: idem as Fig. 1 but the catalyst amount was 60 mg and the reaction period was 2.5 h. | ||
Catalytic reaction at high temperature
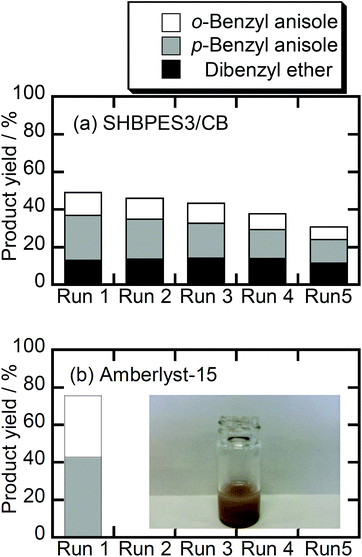
One advantage of the SHBPES as a polymer-based solid acid catalyst is the thermal stability of the poly(ether sulfone) backbone; therefore, a higher operating temperature can be expected compared to Amberlyst®-15. In this context, the Friedel–Crafts alkylation of anisole with benzyl alcohol was studied using the SHBPES3/CB as illustrated in Scheme 2.Fig. 5 summarizes the results of the Friedel–Crafts alkylation of anisole with benzyl alcohol over SHBPES3/CB and Amberlyst®-15. A blank test under the same reaction conditions but without the catalyst showed only trace amounts of reaction products. In contrast, SHBPES3/CB showed a certain catalytic activity for this reaction. Furthermore, the catalyst was successfully collected after the reaction and used for the following four runs, although the performance slightly degraded as the run number increased. Amberlyst®-15 also showed catalytic activity under the same conditions, but the reaction mixture became turbid immediately at the early stage of the reaction, and it was impossible to collect the catalyst. Probably, Amberlyst®-15 was decomposed in the present reaction conditions and worked as a homogeneous catalyst. These experimental results clearly suggest that the stability of the SHBPES/CB catalyst is much better than that of Amberlyst®-15 under such a high temperature.
Interestingly, the product selectivities are slightly different between SHBPES3/CB and Amberlyst®-15. SHBPES3/CB produced dibenzyl ether whereas Amberlyst®-15 did not. This different selectivity might derive from the difference in the solubility of the reactants in the polymer matrix. This result suggests that the HBPES derived solid acid catalyst might exhibit a molecular sieving effect if the polymer matrix and the reaction were appropriately designed.
Conclusions
Sulfonic acid functionalized hyperbranched poly(ether sulfone) (SHBPES) has been investigated as a novel type of solid acid catalyst. Various molecular weights of SHBPESs were tested for the esterification reaction between acetic acid and 1-butanol. The SO3H terminal groups of the HBPESs work as catalytically active sites for the esterification, but all tested SHBPESs are totally or partially soluble under the current reaction conditions. To overcome the solubility problem, SHBPES was grafted onto a carbon black and those materials show fairly good catalytic activity and promising recyclability. It should be noted that SHBPES has been immobilized without any hydrolyzable bonds, which will contribute to high chemical stability under acidic conditions. As a model reaction at a high operating temperature, the Friedel–Crafts alkylation of anisole at 130 °C was studied and it has been revealed that SHBPES3/CB exhibits much better stability than Amberlyst®-15. Indeed, the mass catalytic activity of the current material is not as good as those of the state-of-the-art catalysts; however, it will be much improved if the concentration of acidic sites could be increased. Further studies are required to increase the concentration of acidic sites and expand the applicability of these catalysts to other acid catalyzed chemical reactions.Acknowledgements
This study was supported by JSPS KAKENHI (26870183). The TEM analysis was performed in the Center for Advanced Materials Analysis in Tokyo Institute of Technology. The authors thank Dr Atsushi Takagaki (The University of Tokyo) for valuable instruction about solid acid catalysts and Mr Masatomo Mikuni and Mr Masaki Tomita for technical assistance. M.K. was supported by Visiting Professorship for Senior International Scientists from Chinese Academy of Science.Notes and references
- Y. B. Huang and Y. Fu, Green Chem., 2013, 15, 1095 RSC.
- T. Okuhara, Chem. Rev., 2002, 102, 3641 CrossRef CAS PubMed.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955 CrossRef CAS PubMed; S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, J. Am. Chem. Soc., 2008, 130, 12787 CrossRef PubMed; M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef PubMed.
- M. A. Harmer and Q. Sun, Appl. Catal., A, 2001, 221, 45 CrossRef CAS.
- T. Okayasu, K. Saito, H. Nishide and M. T. W. Hearn, Chem. Commun., 2009, 4708 RSC; T. Okayasu, K. Saito, H. Nishide and M. T. W. Hearn, Green Chem., 2010, 12, 1981 RSC.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587 CrossRef CAS.
- F. Wang, M. Hickner, Y. S. Kim, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231 CrossRef CAS.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183 CrossRef CAS PubMed.
- M. Jikei and M. Kakimoto, Prog. Polym. Sci., 2001, 26, 1233 CrossRef CAS.
- M. Kakimoto, S. J. Grunzinger and T. Hayakawa, Polym. J., 2010, 42, 697 CrossRef CAS.
- K. Matsumoto and M. Ueda, Chem. Lett., 2006, 35, 1196 CrossRef CAS.
- S. J. Grunzinger, T. Hayakawa and M. Kakimoto, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4785 CrossRef CAS; S. J. Grunzinger, M. Watanabe, K. Fukagawa, R. Kikuchi, Y. Tominaga, T. Hayakawa and M. Kakimoto, J. Power Sources, 2008, 175, 120 CrossRef PubMed.
- C. Gao, S. Muthukrishnan, W. W. Li, J. Y. Yuan, Y. Y. Xu and A. H. E. Muller, Macromolecules, 2007, 40, 1803 CrossRef CAS.
- C. M. Homenick, G. Lawson and A. Adronov, Polym. Rev., 2007, 47, 265 CrossRef CAS.
- R. K. Layek and A. K. Nandi, Polymer, 2013, 54, 5087 CrossRef CAS PubMed.
- P. Liu, Eur. Polym. J., 2005, 41, 2693 CrossRef CAS PubMed.
- T. Q. Liu, R. Casado-Portilla, J. Belmont and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4695 CrossRef CAS.
- N. G. Sahoo, S. Rana, J. W. Cho, L. Li and S. H. Chan, Prog. Polym. Sci., 2010, 35, 837 CrossRef CAS PubMed.
- G. Sakellariou, D. Priftis and D. Baskaran, Chem. Soc. Rev., 2013, 42, 677 RSC.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357 CrossRef CAS PubMed.
- C. C. Wang, Z. X. Guo, S. K. Fu, W. Wu and D. B. Zhu, Prog. Polym. Sci., 2004, 29, 1079 CrossRef CAS PubMed.
- H. J. Lee, S. W. Han, Y. D. Kwon, L. S. Tan and J. B. Baek, Carbon, 2008, 46, 1850 CrossRef CAS PubMed.
- F. Liu, W. Kong, C. Qi, L. Zhu and F.-S. Xiao, ACS Catal., 2012, 2, 565 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)