Effects of herbal infusions, tea and carbonated beverages on alcohol dehydrogenase and aldehyde dehydrogenase activity
Sha
Li
,
Li-Qin
Gan
,
Shu-Ke
Li
,
Jie-Cong
Zheng
,
Dong-Ping
Xu
and
Hua-Bin
Li
*
Guangdong Provincial Key Laboratory of Food, Nutrition and Health, Department of Nutrition, School of Public Health, Sun Yat-Sen University, Guangzhou 510080, China. E-mail: lihuabin@mail.sysu.edu.cn; Fax: +86-20-87330446; Tel: +86-20-87332391
First published on 25th September 2013
Abstract
Various alcoholic beverages containing different concentrations of ethanol are widely consumed, and excessive alcohol consumption may result in serious health problems. The consumption of alcoholic beverages is often accompanied by non-alcoholic beverages, such as herbal infusions, tea and carbonated beverages to relieve drunk symptoms. The aim of this study was to supply new information on the effects of these beverages on alcohol metabolism for nutritionists and the general public, in order to reduce problems associated with excessive alcohol consumption. The effects of 57 kinds of herbal infusions, tea and carbonated beverages on alcohol dehydrogenase and aldehyde dehydrogenase activity were evaluated. Generally, the effects of these beverages on alcohol dehydrogenase and aldehyde dehydrogenase activity are very different. The results suggested that some beverages should not be drank after excessive alcohol consumption, and several beverages may be potential dietary supplements for the prevention and treatment of problems related to excessive alcohol consumption.
1. Introduction
Alcohol consumption has been commonplace since pre-historic times, and various alcoholic beverages are widely consumed worldwide nowadays. In moderation, alcohol consumption can exert a range of protective effects. People who drink regularly and moderately, in contrast to non-drinkers, tend to have better insulin sensitivity, a lower risk of vascular disorders, diabetes and dementia, and reduced overall mortality.1–3 Moreover, women who drink moderately may be at a lower risk of weight gain.4 However, excessive consumption of alcohol, whether acute or chronic, is often associated with a tremendous burden of disease and dysfunction; not only alcoholic hepatitis and its associated diseases cirrhosis and hepatocarcinoma, but also a range of other disorders including nausea, dyspepsia, malabsorption of nutrients, pancreatitis, cardiomyopathy, hypertension, strokes, and fetal alcohol syndrome.2,5,6 The primary mediator of these adverse effects appears to be the first metabolite of ethanol, acetaldehyde.7,8 Ethanol is metabolized by a 2-step process in which alcohol dehydrogenase (ADH) oxidizes ethanol to acetaldehyde, which is further oxidized to acetate by aldehyde dehydrogenase (ALDH). Acetaldehyde has been incriminated as an etiological factor in the pathogenesis of lesions and tumors of the large bowel.2,9 The metabolic, pharmacological and toxicological effects of ethanol depend on the duration of exposure, and the concentrations of ethanol and accumulated acetaldehyde in body fluids and tissues.7 Studies have confirmed that several factors may affect the extent of the first-pass metabolism of ethanol, such as food consumption, genetic polymorphism of alcohol-metabolizing enzymes, or medications which interfere with the activity of the metabolizing enzymes and/or with the absorption of ethanol.2,10,11 ADH and ALDH are the principal enzymes responsible for the metabolism of ethanol in humans. If some foods (such as non-alcoholic beverages) could change the activities of ADH and ALDH, they would increase/reduce the toxicity of ethanol and aldehyde to humans.The consumption of alcoholic beverages is often accompanied by non-alcoholic beverages, such as herbal infusions, tea and carbonated beverages to relieve drunk symptoms. Traditionally, tea and herbal infusions were consumed only before drinking. Nowadays, many varieties of tea and herbal infusions have been produced and sold commercially. A special type of herbal infusion is called cool tea (Liang cha in Chinese), which originated from South China. The cool tea is made from different kinds of herbs, and has been drunk as a beverage for hundreds of years. It have been reported that the cool tea has the efficacies of clearing away heat, detoxification, dewetting, moistening the lungs, stopping thirst, relieving fever, alleviating pain, restoring strength, modulating immunity, and antioxidant and anticancer properties.12–15 Tea consumption is also associated with reduced risks of cardiovascular disease and cancers. Tea and herbal infusions are popular beverages, and widely consumed in China and many other places in the world. In addition, carbonated beverages are widely drunk in the world due to their special flavors, specifically the effect of carbonation on perception.16
Despite their widespread use, the effects of herbal infusions, tea and carbonated beverages on ADH and ALDH activity have not been evaluated. Furthermore, an increasing number of accidents are being reported, where there is concern that the alcoholism was exacerbated when some foods were consumed after drinking excessive amounts of alcohol. This could be because some foods (such as beverages) possess the ability to decrease/increase the activity of alcohol-metabolizing enzymes. Thus, it is worth investigating scientifically if some beverages are inappropriate to drink after excessive alcohol consumption. In addition, it is worth attempting to look for some effective beverages capable of reducing the effects of alcohol. Therefore, the aim of this study was to systematically evaluate the effects of 57 kinds of beverages on alcohol metabolism, to investigate how the ADH and ALDH activity is influenced by these beverages, and to supply new information on the effects of these beverages on alcohol metabolism for nutritionists and the general public to reduce the harm from excessive alcohol consumption.
2. Materials and methods
2.1. Chemicals and samples
Alcohol dehydrogenase, aldehyde dehydrogenase and NAD+ were purchased from Sigma Chemical Co. (St. Louis, Missouri, USA). Ethanol, acetaldehyde, sodium pyrophosphate, pyrazole, acetic acid and sodium hydroxide were purchased from Tianjing Chemical Factory (Tianjing, China). Acetaldehyde was re-distilled before use. All of the other chemicals and solvents used in this study were of analytical grade.The 57 herbal infusions, tea and carbonated beverages were bought from local markets in Guangzhou, China, which are commercial preparations and in the form of tins containing an aqueous solution. The various beverages were centrifuged at 4200g for 20 min, and then stored at 4 °C for the evaluation of the activities within 1–2 days.
2.2. Assays of ADH and ALDH activities
ADH activity was determined by the modified Valle & Hoch method.17 In brief, 1.5 mL pyrophosphate buffer (0.1 M, pH 8.8), 0.1 mL of 0.25 U mL−1 ADH, 0.5 mL ethanol (11.5%, v/v), and 0.1 mL of the beverage sample were mixed at 25 °C, and then 1.0 mL 0.01 M NAD+ was added to initiate the reaction. The absorbance was immediately measured at 340 nm, and was measured again after 15 min.ALDH activity was determined by the modified Blair & Bodley method.18 In brief, 1.6 mL pyrophosphate buffer (0.1 M, pH 9.5), 0.1 mL of 0.25 U mL−1 ADH, 0.1 mL of 0.1 M acetaldehyde, 0.1 mL of 0.01 M pyrazole, and 0.1 mL of the beverage sample were mixed at 30 °C, and then 1.0 mL of 3.6 mM NAD+ was added to initiate the reaction. The absorbance was immediately measured at 340 nm, and was measured again after the mixture was warmed at 30 °C for 15 min.
The absorbance in the absence of ethanol or acetaldehyde was subtracted as the blank. One milliunit (mU) of the enzyme activity of ADH or ALDH corresponds to 1 nmol NADH produced per minute, based on the extinction coefficient of 6.22 mM−1 cm−1 for NADH at 340 nm. The activity was expressed as a percentage compared to the control.
2.3. Statistical analysis
All of the experiments were carried out in triplicate, and the results were expressed as the mean ± SD (standard deviation). Statistical analysis was performed using SPSS 13.0 and Excel 2003. Differences between the means of the data for the different beverage samples were compared by the least significant difference (LSD) test. A p value of less than 0.05 was considered to be statistically significant.3. Results and discussion
Various alcoholic beverages are widely drunk in the world. They contain different concentrations of ethanol, for example, strong wine (50–65%), grape wine (10–15%) and beer (2–5%). However, the excessive consumption of alcohol could result in social problems (such as traffic accidents) and various health problems (such as nausea and colorectal cancer).19 When excessive ethanol is consumed, acetaldehyde appears likely to be the key mediator of the subsequent hangover.20,21 Hence, effective hangover prevention will either suppress levels of acetaldehyde following ethanol consumption, or will antagonize the certain toxic organ-based effects of this compound.10,22 When excessive alcohol is drunk, humans usually drink a lot of non-alcoholic beverages in the anticipation that ethanol and its metabolites will be expelled out of the body through the urine. In this study, the effects of 57 kinds of herbal infusions, tea and carbonated beverages on ADH and ALDH activity were evaluated systematically. These beverages were obtained from markets in Guangzhou, and represent the main categories of the beverages made in China, including 40 kinds of herbal infusions, 12 kinds of tea infusions, and 5 kinds of carbonated beverages (Table 1).| No. | Name | Name in Chinese | ADH (%) | Difference of ADHb | ALDH (%) | Difference of ALDHb |
|---|---|---|---|---|---|---|
| a “H”: herbal infusion; “T”: tea infusion; “C”: carbonated beverage; “*”: difference between the sample and blank was statistically significant (p < 0.05). b Difference among the different samples was not statistically significant (p > 0.05). | ||||||
| 1H | Wu hua qu shi cha |

|
−66.6 ± 1.4 | * | −60.6 ± 2.1 | *, 13H, 49T |
| 2H | Qing yan li hou cha |

|
−83.7 ± 1.8 | *, 19H | −132.4 ± 3.2 | * |
| 3H | Qing re jie biao cha |

|
−87.8 ± 0.8 | * | −95.8 ± 1.3 | *, 5H, 6H, 7H, 10H, 11H, 14H, 15H, 16H, 18H, 20H, 25H, 38H, 44T, 45T, 47T, 51T |
| 4H | Qing re an chuang cha |

|
−41.7 ± 0.4 | *, 9H | −108.5 ± 1.1 | *, 9H, 12H, 26H, 31H |
| 5H | Zhi ke hua tan cha |
|
−64.0 ± 0.9 | *, 6H, 22H, 23H | −91.5 ± 1.7 | *, 3H, 6H, 10H, 11H, 17H, 18H, 25H, 38H, 41H, 42T, 44T, 51T |
| 6H | Qing gan cha |
|
−64.1 ± 1.2 | *, 5H, 22H, 23H | −91.6 ± 2.5 | *, 3H, 5H, 10H, 11H, 17H, 18H, 25H, 38H, 41H, 42T, 44T, 51T |
| 7H | Luo han guo cha |

|
−14.7 ± 0.7 | *, 21H, 35H, 48T, 49T | −100.0 ± 3.3 | *, 3H, 11H, 12H, 14H, 15H, 16H, 20H, 38H, 45T, 47T, 51T, 52T |
| 8H | Luo shen hua cha |
|
7.6 ± 0.1 | *, 17H, 20H, 29H, 32H, 53T, 55C | −84.5 ± 3.2 | *, 17H, 18H, 25H, 37H, 41H, 42T |
| 9H | Deng lao liang cha (guan zhuang) |

|
39.9 ± 0.7 | * | −110.7 ± 4.5 | *, 4H, 20H, 26H, 31H |
| 10H | Jia duo bao liang cha |

|
−21.6 ± 0.6 | *, 15H, 16H, 51T, 52T | −93.0 ± 2.9 | *, 3H, 5H, 6H, 11H, 15H, 16H, 17H, 18H, 20H, 25H, 38H, 44T, 45T, 47T, 51T |
| 11H | Qing ku lv liang cha |

|
−5.2 ± 0.1 | *, 30H, 31H, 34H, 45T, 54C | −95.8 ± 1.4 | *, 3H, 5H, 6H, 7H, 10H, 14H, 15H, 16H, 18H, 20H, 25H, 38H, 44T, 45T, 47T, 51T |
| 12H | He qi zheng liang cha |
|
3.6 ± 0.2 | * | −104.2 ± 6.1 | *, 4H, 15H, 16H, 20H, 26H, 45T, 47T, 52T |
| 13H | Qing ku ling meng liang cha |

|
21.7 ± 1.1 | *, 24H | −57.7 ± 2.0 | *, 1H, 34H, 49T, 50T |
| 14H | Wang lao ji liang cha (hong guan) |

|
0.8 ± 0.0 | 42T, 46T, 47T, 57C | −100.0 ± 3.7 | *, 3H, 7H, 11H, 12H, 14H, 15H, 16H, 20H, 38H, 45T, 47T, 51T, 52T |
| 15H | Wang lao ji liang cha (lv he) |

|
−20.1 ± 0.8 | *, 10H, 16H, 52T | −98.6 ± 4.6 | *, 3H, 7H,10H, 11H, 12H, 14H, 16H, 20H, 38H, 45T, 47T, 51T, 52T |
| 16H | Bao qing tang si ji liang cha |

|
−19.0 ± 1.3 | *, 10H, 15H, 51T, 52T | −98.6 ± 2.9 | *, 3H, 10H, 11H, 12H, 14H, 15H, 20H, 38H, 45T, 47T, 52T |
| 17H | Liang cha yin liao |
|
8.4 ± 0.8 | *, 8H, 29H, 32H, 55C | −87.3 ± 4.5 | *, 5H, 6H, 8H, 10H, 18H, 25H, 37H, 41H, 42T, 44T |
| 18H | Mao gen zhu zhe shui |
|
−1.5 ± 0.1 | 40H, 46T, 47T | −90.1 ± 6.6 | *, 3H, 5H, 6H, 8H, 10H, 11H, 17H, 25H, 38H, 41H, 42T, 44T |
| 19H | Ban sha |
|
−84.9 ± 5.3 | *, 2H | −49.3 ± 2.5 | *, 34H, 50T, 54C |
| 20H | Tian wei ban sha |

|
6.2 ± 0.3 | *, 8H, 29H, 39H, 53T, 55C | −98.6 ± 7.7 | *, 3H, 7H,10H, 11H, 12H, 14H, 15H, 16H, 38H, 45T, 47T, 51T, 52T |
| 21H | Ju hua xue li cha |

|
−13.8 ± 0.6 | *, 7H, 35H, 38H, 43T, 44T | −71.8 ± 0.5 | *, 22H, 23H, 35H, 43T, 46T, 48T |
| 22H | Luo han guo hua cha |

|
−64.5 ± 4.0 | *, 5H, 6H, 23H | −73.2 ± 1.9 | *, 21H, 23H, 35H, 43T, 46T, 48T |
| 23H | Jin yin hua cha |
|
−63.9 ± 4.2 | *, 5H, 6H, 22H | −74.6 ± 5.8 | *, 21H, 22H, 35H, 43T, 46T, 48T |
| 24H | Yi ren qu shi cha |

|
20.1 ± 0.8 | *, 13H | −25.4 ± 1.0 | *, 28H, 32H, 40T, 56C |
| 25H | Huo ma ren |

|
36.0 ± 2.1 | * | −90.1 ± 1.5 | *, 3H, 5H, 6H, 10H, 11H, 17H, 18H, 38H, 41H, 42T, 44T |
| 26H | Shen hui liang cha wang |

|
11.8 ± 0.4 | *, 27H | −109.8 ± 7.0 | *, 4H, 9H, 12H, 31H |
| 27H | Bao qing tang xue li ju hua cha (ping zhuang) |
|
11.2 ± 0.1 | *, 26H | −14.1 ± 0.2 | *, 29H, 30H, 33H |
| 28H | Bao qing tang xue li ju hua cha (he zhuang) |
|
14.1 ± 0.7 | *, 41H, 56C | −21.1 ± 1.1 | *, 24H, 32H, 33H |
| 29H | Bai yi ju hua yin liao |
|
7.5 ± 0.3 | *, 8H, 17H, 20H, 32H, 36H, 53T, 55C | −14.0 ± 0.5 | *, 27H, 30H, 33H |
| 30H | Shen hui dong gua cha |
|
−7.0 ± 0.1 | *, 11H, 31H, 34H, 45T, 50T, 54C | −11.3 ± 0.2 | *, 27H, 29H, 33H |
| 31H | Lao weng liang cha cao |

|
−6.0 ± 0.2 | *, 11H, 30H, 34H, 45T, 50T, 54C | −112.7 ± 3.5 | *, 4H, 9H, 26H |
| 32H | Shen hui mao gen zhe zhi yin liao |

|
8.8 ± 0.4 | *, 8H, 17H, 29H, 55C | −25.4 ± 1.1 | *, 24H, 28H, 40H, 56C |
| 33H | Xia guang mao gen zhe zhi yin liao |

|
2.9 ± 0.1 | *, 42T | −15.5 ± 0.6 | *, 27H, 28H, 29H, 30H |
| 34H | Shen hui ju hua zhi wu yin liao |

|
−6.3 ± 0.1 | *, 11H, 30H, 31H, 45T, 50T, 54C | −53.5 ± 5.1 | *, 13H, 19H, 50T |
| 35H | Shen hui qing liang cha zhi wu yin liao |

|
−14.3 ± 1.1 | *, 7H, 21H, 38H, 43T, 48T | −70.4 ± 3.4 | *, 21H, 22H, 23H, 43T |
| 36H | Bai yi qing liang cha |

|
5.8 ± 0.2 | *, 8H, 20H, 39H, 53T, 55C | 8.5 ± 0.7 | *, 34H, 39H |
| 37H | Suan mei tang |
|
17.2 ± 1.8 | * | −81.7 ± 6.9 | *, 8H, 17H, 41H, 42T |
| 38H | Yang xie cheng qing liang shuang |
|
−12.5 ± 0.9 | *, 21H, 35H, 43T, 44T | −95.8 ± 2.3 | *, 3H, 5H, 6H, 7H, 10H, 11H, 14H, 15H, 16H, 18H, 20H, 25H, 44T, 45T, 47T, 51T, 52T |
| 39H | Yang xie cheng ma ti shuang |
|
5.5 ± 0.2 | *, 11H, 20H, 30H, 31H, 34H, 36H, 45T, 50T, 53T, 55C | 8.4 ± 0.3 | *, 36H, 45T |
| 40H | Wang lao ji lian zi lv dou shuang yin liao |
|
−1.9 ± 0.1 | *, 18H, 46T | −29.6 ± 1.7 | *, 24H, 32H, 56C |
| 41H | Qu chen shi sha shi |
|
14.7 ± 1.4 | *, 28H, 56C | −85.9 ± 6.0 | *, 5H, 6H, 8H, 18H, 17H, 25H, 37H, 42T, 44T |
| 42T | Kang shi fu bing hong cha |
|
1.6 ± 0.1 | 14H, 33H, 47T, 57C | −85.9 ± 7.5 | *, 5H, 6H, 8H, 18H, 17H, 25H, 37H, 41H, 44T |
| 43T | Tong yi bing hong cha |
|
−12.5 ± 0.2 | *, 21H, 35H, 38H, 44T | −71.8 ± 1.3 | *, 21H, 23H, 22H, 35H, 46T, 48T |
| 44T | Tong yi lv cha |
|
−12.3 ± 0.1 | *, 21H, 38H, 43T | −91.5 ± 8.0 | *, 3H, 5H, 6H, 10H, 11H, 17H, 18H, 25H, 38H, 41H, 42T, 51T |
| 45T | Kang shi fu tie guan yin cha |
|
−5.8 ± 0.4 | *, 11H, 30H, 31H, 34H, 50T, 54C | −98.6 ± 5.5 | *, 3H, 7H, 10H, 11H, 12H, 14H, 15H, 16H, 20H, 38H, 47T, 51T, 52T |
| 46T | Kang shi fu long jin cha |

|
−0.6 ± 0.0 | 14H, 18H, 40H, 47T, 57C | −77.5 ± 3.2 | *, 21H, 22H, 23H, 43T, 48T |
| 47T | Yuan ye dian hong hong cha |
|
0.1 ± 0.0 | 14H, 18H, 42T, 46T, 57C | −98.6 ± 2.2 | *, 3H, 7H, 10H, 11H, 12H, 14H, 15H, 16H, 20H, 38H, 45T, 51T, 52T |
| 48T | Kang shi fu gan chun lv cha |
|
−15.7 ± 0.9 | *, 7H, 35H, 49T | −77.4 ± 5.1 | *, 21H, 22H, 23H, 43T, 46T |
| 49T | Kang shi fu wu long ming cha |
|
−16.4 ± 0.1 | *, 7H, 48T | −62.0 ± 1.5 | *, 1H, 13H |
| 50T | Kang shi fu mo li qing cha |

|
−7.3 ± 0.7 | *, 30H, 31H, 34H, 45T, 54C | −53.5 ± 4.1 | *, 13H, 19H, 34H |
| 51T | Dong fang shu ye wu long cha |
|
−18.2 ± 0.1 | *, 16H, 49T, 52T | −97.2 ± 5.7 | *, 3H, 5H, 6H, 7H, 10H, 11H, 14H, 15H, 16H, 20H, 38H, 44T, 45T, 47T, 52T |
| 52T | Dong fang shu ye hong cha |
|
−18.3 ± 1.5 | *, 15H, 16H, 51T | −101.4 ± 6.2 | *, 3H, 7H, 11H, 12H, 14H, 15H, 16H, 20H, 38H, 45T, 47T, 51T |
| 53T | Wa ha ha bing hong cha |
|
6.3 ± 0.4 | *, 8H, 20H, 29H, 36H, 39H, 55C | −38.0 ± 2.9 | * |
| 54C | Hui yi su da shui |

|
−5.7 ± 0.5 | *, 11H, 30H, 31H, 34H, 36H, 45T, 50T | 49.3 ± 3.3 | * |
| 55C | Xue bi |

|
7.3 ± 0.6 | *, 8H, 17H, 20H, 29H, 32H, 36H, 39H, 53T | 28.2 ± 0.7 | * |
| 56C | Ke kou ke le |

|
13.9 ± 0.8 | *, 28H, 41H | −28.2 ± 1.5 | *, 24H, 32H, 40H |
| 57C | Bai shi ke le |

|
0.6 ± 0.0 | 14H, 42T, 46T, 47T | 0.1 ± 0.0 | — |
As can be seen from Table 1, these beverages could be categorized into four groups according to their effects on ADH and ALDH activity: (1) increase ADH activity and ALDH activity, (2) increase ADH activity and decrease ALDH activity, (3) decrease ADH activity and ALDH activity, and (4) decrease ADH activity and increase ALDH activity. In addition, the difference between most samples and the blank, as well as the difference among most samples, were statistically significant (Table 1).
3.1. The beverages which increase ADH activity and ALDH activity
Four out of the 57 kinds of beverages could increase ADH activity and also increase ALDH activity (Table 1). Xue bi weakly increases ADH activity, and markedly increases ALDH activity. That is, ethanol is slowly metabolized to the more toxic aldehyde, but the aldehyde can be rapidly metabolized to non-toxic acetic acid. ALDH converts acetaldehyde to acetate in a reaction which does not generate oxidants. Acetate appears to be innocuous in the moderate concentrations generated during alcohol metabolism; indeed, it is suspected that acetate may mediate many of the protective health effects associated with the regular moderate consumption of alcohol.23 Measures which boost the activity of ALDH have the potential to mitigate the adverse effects of alcohol consumption by minimizing tissue exposure to acetaldehyde.10 Thus, this beverage is beneficial in reducing the harm from excessive alcohol consumption. Moreover, ALDH, a detoxifying enzyme responsible for oxidizing intracellular aldehydes, plays an important role in multiple biological activities, including drug resistance, cell differentiation and oxidative metabolism.24 The activation of ALDH by xue bi may be beneficial for our health even without alcohol consumption. It has been reported that taurine, which is added to many soft drinks, can promote the efficient elimination of acetaldehyde by influencing the effects of ALDH.10,25 This suggests that some compounds which are present in xue bi may have the same effect on ALDH as taurine. In addition, bai yi qing liang cha, yang xie cheng ma ti shuang, and bai shi ke le could weakly increase the ADH and ALDH activity, and would not have significant effects on the metabolism of ethanol and aldehyde.3.2. The beverages which increase ADH activity and decrease ALDH activity
Twenty-one out of the 57 kinds of beverages could increase ADH activity and decrease ALDH activity (Table 1). Deng lao liang cha (Guan zhuang), and huo ma ren significantly increase ADH activity, but markedly decrease ALDH activity. Taraxerone has been identified from Sedum sarmentosum, and a study was performed to evaluate the effects of taraxerone on enhancing the activity of ADH and ALDH via in vitro and in vivo investigations. It was demonstrated that ADH and ALDH activity was enhanced by taraxerone via the increase of ADH and ALDH gene expression, thus the plasma alcohol and acetaldehyde levels were efficiently reduced by taraxerone treatment in a concentration-dependent manner.26 These infusions were made from different kinds of herbs and medical plants, which contain numerous and complicated bioactive compounds. Some of them might act like taraxerone, or taraxerone may be one of the components. However, because the components of these infusions are complex, some other ingredients might possess an inactivation effect towards ALDH.These infusions could accelerate ethanol metabolism to more toxic aldehydes, but the metabolism of aldehyde to non-toxic acetic acid is seriously prohibited. Aldehydes would accumulate and result in a series of health problems. Oxidative stress and other toxic consequences of acetaldehyde exposure appear to be primarily responsible for the many adverse health effects of chronic alcohol abuse, and also for the increased cancer risks associated with moderate alcohol consumption.27,28 Therefore, these beverages should not be drunk by humans in combination with excessive alcohol consumption. The other beverages in this group are also not beneficial to humans who consume alcohol excessively because they could more or less increase the ADH activity and significantly decrease the ALDH activity. It would be better if humans with excessive alcohol consumption do not drink these beverages simultaneously.
3.3. The beverages which decrease ADH activity and ALDH activity
Thirty-one out of the 57 kinds of beverages could decrease ADH activity and also decrease ALDH activity (Table 1). Qing re jie biao cha, and qing yan li hou cha, could markedly decrease the ADH and ALDH activity. Green tea and tea products have been proven to be rich in catechins and flavonoidal polyphenols by numerous quantifying techniques. In one study, it was demonstrated that catechins and flavonoids, including four new quercetin glycosides isolated from tea leaves, showed strong yeast ADH-inhibitory activities.29 With regard to this section, the results obtained here are consistent with the results reported in the literature.29 The metabolism of ethanol to aldehyde is seriously prohibited by these beverages, and the drunk symptoms of humans with excessive alcohol consumption would not be relieved. In addition, the metabolism of toxic aldehydes to non-toxic acetic acid is also seriously prohibited, which is not beneficial in reducing the harm from excessive alcohol consumption. Therefore, it is better not drink these beverages with excessive alcohol consumption. For other beverages of this group, they could more or less decrease ADH activity and significantly decrease ALDH activity, which would prohibit the metabolism of ethanol and aldehyde, and are not also beneficial to humans with excessive alcohol consumption. Thus, it would be better if humans with excessive alcohol consumption do not drink these beverages simultaneously.3.4. The beverages which decrease ADH activity and increase ALDH activity
Only 1 out of the 57 kinds of beverages could weakly decrease ADH activity and markedly increase ALDH activity (Table 1). That is, the metabolism of ethanol to more toxic aldehydes is weakly prohibited, but aldehydes could rapidly be metabolized to non-toxic acetic acid. Beverages which boost the activity of ALDH have the potential to mitigate the adverse effects of alcohol consumption by minimizing tissue exposure to acetaldehyde. Thus, this beverage (hui yi su da shui) is very beneficial in reducing the harm from excessive alcohol consumption. Hui yi su da shui is a type of weak alkali soda drink, which contains some flavoring additives and sugar. A specific additive may be the bioactive compound responsible for the activation of ALDH, which is worthy of further study.3.5. The relationship between the ADH and ALDH activity influenced by these beverages
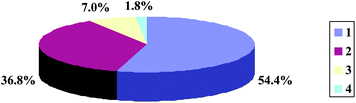
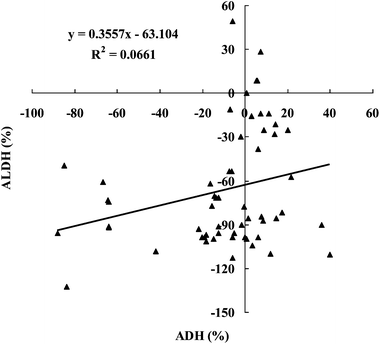
The fifty-seven kinds of beverages have been categorized into four groups, according to their effects on ADH and ALDH activity, and their percentages are shown in Fig. 1. As can be seen from Fig. 1, 31 out of 57 beverages (54.4%) decrease ADH activity and ALDH activity, 21 out of 57 beverages (36.8%) increase ADH activity and decrease ALDH activity, 4 out of 57 beverages (7.0%) increase ADH activity and ALDH activity, and 1 out of 57 beverages (1.8%) decreases ADH activity and increases ALDH activity. In addition, the relationship between the ADH and ALDH activities influenced by 57 beverages is shown in Fig. 2. A very weak correlation (R2 = 0.0661) between the ADH and ALDH activity as influenced by the 57 beverages suggests that the components capable of increasing (or decreasing) ADH activity could be different from those which increase (or decrease) ALDH activity. Alternatively, the same component could increase (or decrease) ADH activity, but decrease (or increase) ALDH activity. This is something worth studying further. Disulfiram, an alcohol-aversive drug, is known to be an excellent inhibitor of both ADH and ALDH, and some of its components may possess the ability to decrease the activities of ADH and ALDH simultaneously.30 Meanwhile, a study has confirmed that catechins and flavonoids isolated from the leaves of Camellia sinensis showed strong yeast ADH-inhibitory activities, yet none of the isolated compounds showed any significant ALDH inhibitory,29 which showed that some compounds from plants could influence ADH and ALDH activity.Furthermore, as can be seen from Fig. 1 and 2, many of these beverages decrease ADH activity and a lot of beverages increase ADH activity; most of these beverages decrease ALDH activity and only several beverages increase ALDH activity. That is, 32 out of 57 beverages (56.1%) decrease ADH activity and another 25 (43.9%) increase ADH activity, while 52 out of 57 beverages (91.2%) decrease ALDH activity and another 5 (8.8%) increase ALDH activity. Thus, there are relatively few beverages which can activate ALDH. Studies have indicated that Asians are deficient in ALDH activity.31,32 Hence, it is suggested that effective hangover prevention requires the ability to boost the activity of ALDH, which has the potential to mitigate the adverse effects of alcohol consumption by minimizing tissue exposure to acetaldehyde.10,33,34 Thus, it is very important that several beverages capable of increasing ALDH activity have been screened out.
Usually, ethanol is rapidly metabolized to toxic aldehydes in the human body, but aldehydes would be very slowly metabolized to non-toxic acetic acid. When the metabolism of ethanol to aldehydes is seriously prohibited, drunk symptoms in humans with excessive alcohol consumption would not be relieved. However, if ethanol is rapidly metabolized to aldehydes, but the metabolism of aldehydes to acetic acid is seriously prohibited, a lot of aldehydes would accumulate in the human body, which would result in a series of health problems. Therefore, it would be better if the ADH and ALDH activity could be simultaneously increased, with the increase of ALDH activity more than that of ADH activity, or if the ALDH activity could be markedly increased with a weak prohibition of the ADH activity. Thus, 2 of the 57 beverages studied, xue bi and hui yi su da shui, are suitable for drinking by humans who consume alcohol excessively.
4. Conclusions
The effects of 57 kinds of herbal infusions, tea and carbonated beverages on ADH and ALDH activity were evaluated. Generally, the effects of these beverages on alcohol dehydrogenase and aldehyde dehydrogenase activities are very different, and several beverages could markedly increase/reduce ADH and ALDH activity. The results suggested that some beverages should not be drunk after excessive alcohol consumption, and several beverages may be potential dietary supplements for the prevention of harm, and treatment, from excessive alcohol consumption. This study has supplied new information on the effects of these beverages on alcohol metabolism for nutritionists and the general public, and further studies on the precise compounds and mechanisms responsible for the activity of these beverages is needed.Acknowledgements
This research was supported by the Hundred-Talents Scheme of Sun Yat-Sen University.References
- S. Costanzo, C. A. Di, M. B. Donati, L. Iacoviello and G. G. De, Alcohol consumption and mortality in patients with cardiovascular disease: a meta-analysis, J. Am. Coll. Cardiol., 2010, 55, 1339–1347 CrossRef PubMed.
- C. P. Chiang, C. W. Wu, S. P. Lee, J. L. Ho, S. L. Lee, S. Nieh and S. J. Yin, Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human small intestine, Alcohol.: Clin. Exp. Res., 2012, 36, 2047–2058 CrossRef CAS PubMed.
- M. F. McCarty, The insulin-sensitizing activity of moderate alcohol consumption may promote leanness in women, Med. Hypotheses, 2000, 54, 794 CrossRef CAS PubMed.
- L. Wang, I. M. Lee, J. E. Manson, J. E. Buring and H. D. Sesso, Alcohol consumption, weight gain, and risk of becoming overweight in middle-aged and older women, Arch. Intern. Med., 2010, 170, 453–461 CrossRef PubMed.
- T. Chiba and S. F. Phillips, Alcohol-related diarrhea, Addict. Biol., 2000, 5, 117–125 CrossRef CAS PubMed.
- P. S. Brocardo, J. Gil-Mohapel and B. R. Christie, The role of oxidative stress in fetal alcohol spectrum disorders, Brain Res. Rev., 2011, 67, 209–225 CrossRef CAS PubMed.
- C. J. Eriksson, The role of acetaldehyde in the actions of alcohol (update 2000), Alcohol.: Clin. Exp. Res., 2001, 25, 15S–32S CrossRef CAS.
- V. Vasiliou, A. Pappa and T. Estey, Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism, Drug. Metab. Rev., 2004, 36, 279–299 CrossRef CAS PubMed.
- T. M. Badger, M. J. Ronis, H. K. Seitz, E. Albano, M. Ingelman-Sundberg and C. S. Lieber, Alcohol metabolism: role in toxicity and carcinogenesis, Alcohol.: Clin. Exp. Res., 2003, 27, 336–347 CrossRef PubMed.
- M. F. McCarty, Nutraceutical strategies for ameliorating the toxic effects of alcohol, Med. Hypotheses, 2013, 80, 456–462 CrossRef CAS PubMed.
- M. H. Pittler, J. C. Verster and E. Ernst, Interventions for preventing or treating alcohol hangover: systematic review of randomised controlled trials, Br. Med. J., 2005, 331, 1515–1518 CrossRef PubMed.
- L. Fu, B. T. Xu, R. Y. Gan, Y. Zhang, X. R. Xu, E. Q. Xia and H. B. Li, Total phenolic contents and antioxidant capacities of herbal and tea infusions, Int. J. Mol. Sci., 2011, 12, 2112–2124 CrossRef CAS PubMed.
- F. Li, S. Li, H. B. Li, G. F. Deng, W. H. Ling and X. R. Xu, Antiproliferative activities of tea and herbal infusions, Food Funct., 2013, 4, 530–538 CAS.
- S. Y. Hu, Herbal teas and populace health care in tropical China, Am. J. Chin. Med., 1997, 25, 103–134 CrossRef CAS PubMed.
- Z. B. Dashinamzhilov, P. B. Lubsandorzhieva, K. S. Lonshakova and S. M. Batorova, A hepatoprotective effect of medicinal herbal tea hexacholefit in ethanol-induced liver damage, Patol. Fiziol. Eksp. Ter., 2008, 1, 25–26 Search PubMed.
- A. Saint-Eve, I. Deleris, G. Feron, D. Ibarra, E. Guichard and I. Souchon, How trigeminal, taste and aroma perceptions are affected in mint-flavored carbonated beverages, Food Quality and Preference, 2010, 21, 1026–1033 CrossRef PubMed.
- J. S. Moreb, A. Gabr, G. R. Vartikar, S. Gowda, J. R. Zucali and D. Mohuczy, Retinoic acid down-regulates aldehyde dehydrogenase and increases cytotoxicity of 4-hydroperoxycyclophosphamide and acetaldehyde, J. Pharmacol. Exp. Ther., 2005, 312, 339–345 CrossRef CAS PubMed.
- A. H. Blair and F. H. Bodley, Human liver aldehyde dehydrogenase: Partial purification and properties, Can. J. Biochem., 1969, 47, 265–272 CrossRef CAS.
- K. Husain, Vascular endothelial oxidative stress in alcohol-induced hypertension, Cell. Mol. Biol., 2007, 53, 70–87 CAS.
- G. P. Voulgaridou, I. Anestopoulos, R. Franco, M. I. Panayiotidis and A. Pappa, DNA damage induced by endogenous aldehydes: current state of knowledge, Mutat. Res., 2011, 711, 13–27 CrossRef CAS PubMed.
- S. A. Marchitti, C. Brocker, D. Stagos and V. Vasiliou, Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily, Expert Opin. Drug Metab. Toxicol., 2008, 4, 697–720 CrossRef CAS PubMed.
- V. Vasiliou, A. Pappa and T. Estey, Role of human aldehyde dehydrogenases in endobiotic and xenobiotic metabolism, Drug Metab. Rev., 2004, 36, 279–299 CrossRef CAS PubMed.
- M. F. McCarty, Does regular ethanol consumption promote insulin sensitivity and leanness by stimulating AMP-activated protein kinase?, Med. Hypotheses, 2001, 57, 405–407 CrossRef CAS PubMed.
- R. J. Kim, J. R. Park, K. J. Roh, A. R. Choi, S. R. Kim, P. H. Kim, J. H. Yu, J. W. Lee, S. H. Ahn, G. Gong, J. W. Hwang, K. S. Kang, G. Kong, Y. Y. Sheen and J. S. Nam, High aldehyde dehydrogenase activity enhances stem cell features in breast cancer cells by activating hypoxia-inducible factor-2a, Cancer Lett., 2013, 333, 18–31 CrossRef CAS PubMed.
- S. L. Devi, P. Viswanathan and C. V. Anuradha, Taurine enhances the metabolism and detoxification of ethanol and prevents hepatic fibrosis in rats treated with iron and alcohol, Environ. Toxicol. Pharmacol., 2009, 27, 120–126 CrossRef CAS PubMed.
- C. K. Sung, S. M. Kim, C. J. Oh, S. A. Yang, B. H. Han and E. K. Mob, Taraxerone enhances alcohol oxidation via increases of alcohol dehyderogenase (ADH) and acetaldehyde dehydrogenase (ALDH) activities and gene expressions, Food Chem. Toxicol., 2012, 50, 2508–2514 CrossRef CAS PubMed.
- A. I. Cederbaum, Y. Lu and D. Wu, Role of oxidative stress in alcohol-induced liver injury, Arch. Toxicol., 2009, 83, 519–548 CrossRef CAS PubMed.
- X. Zhang, S. Y. Li, R. A. Brown and J. Ren, Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress, Alcohol, 2004, 32, 175–186 CrossRef CAS PubMed.
- M. M. Manir, J. K. Kim, B. G. Lee and S. S. Moon, Tea catechins and flavonoids from the leaves of Camellia sinensis inhibit yeast alcohol dehydrogenase, Bioorg. Med. Chem., 2012, 20, 2376–2381 CrossRef CAS PubMed.
- Y. Shen, K. Lindemeyer, C. Gonzalez, X. M. Shao, I. Spingelman, R. W. Olsen and J. Liang, Dihydromyricetin as a novel anti-alcohol intoxication medication, J. Neurosci., 2012, 32, 390–401 CrossRef CAS PubMed.
- T. L. Wall, S. M. Horn, M. L. Johnson, T. L. Smith and L. G. Carr, Hangover symptoms in Asian Americans with variations in the aldehyde dehydrogenase (ALDH2) gene, J. Stud. Alcohol., 2000, 61, 13–17 CAS.
- R. Penning, N. M. Van, L. A. Fliervoet, B. Olivier and J. C. Verster, The pathology of alcohol hangover, Curr. Drug Abuse Rev., 2010, 3, 68–75 CrossRef CAS.
- S. Singh, C. Brocker, V. Koppaka, Y. Chen, B. C. Jackson, A. Matsumoto, D. C. Thompson and V. Vasiliou, Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress, Free Radical Biol. Med., 2013, 56, 89–101 CrossRef CAS PubMed.
- N. E. Sladek, Human aldehyde dehydrogenases: potential pathological, pharmacological, and toxicological impact, J. Biochem. Mol. Toxicol., 2003, 17, 7–23 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |