Quantitative evaluation of the ability of ionic liquids to offset the cold-induced unfolding of proteins†
Abstract
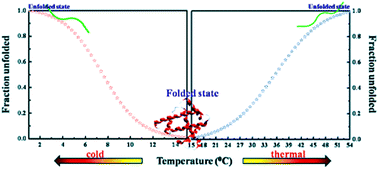
Significant non-reversible two-state denaturation was observed for proteins such as myoglobin (Mb) and α-chymotrypsin (CT) with decreasing temperature in the presence of 1-butyl-3-methylimidazolium-based ([C4mim]+X−) ionic liquids (ILs) with various anions (X−). Interestingly, for the first time, ILs having acetate and bromide anions were proven to counteract the cold-induced unfolding of proteins.

 Please wait while we load your content...
Please wait while we load your content...