Pushing the detection limit of infrared spectroscopy for structural analysis of dilute protein samples†
Abstract
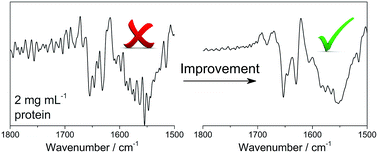
Fourier-transform infrared spectroscopy is a powerful and versatile tool to investigate the structure and dynamics of proteins in solution. The intrinsically low extinction coefficient of the amide I mode, the main structure-related oscillator, together with the high infrared absorptivity of aqueous media, requires that proteins are studied at high concentrations (>10 mg L−1). This may represent a challenge in the study of aggregation-prone proteins and peptides, and questions the significance of structural data obtained for proteins physiologically existing at much lower concentrations. Here we describe the development of a simple experimental approach that increases the detection limit of protein structure analysis by infrared spectroscopy. Our approach relies on custom-made filters to isolate the amide I region (1700–1600 cm−1) from irrelevant spectral regions. The sensitivity of the instrument is then increased by background attenuation, an approach consisting in the use of a neutral density filter, such as a non-scattering metal grid, to attentuate the intensity of the background spectrum. When the filters and grid are combined, a 2.4-fold improvement in the noise level can be obtained. We have successfully tested this approach using a highly diluted solution of pyruvate kinase in deuterated medium (0.2% w/v), and found that it provides spectra of a quality comparable to those recorded with a 10-fold higher protein concentration.


 Please wait while we load your content...
Please wait while we load your content...