Quadruplex priming amplification for the detection of mRNA from surrogate patient samples†
Abstract
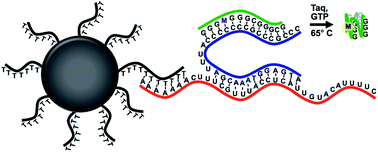
Simple and rapid methods for detecting mRNA biomarkers from patient samples are valuable in settings with limited access to laboratory resources. In this report, we describe the development and evaluation of a self-contained assay to extract and quantify mRNA biomarkers from complex samples using a novel nucleic acid-based molecular sensor called quadruplex priming amplification (QPA). QPA is a simple and robust isothermal nucleic acid amplification method that exploits the stability of the G-quadruplex nucleotide structure to drive spontaneous strand melting from a specific DNA template sequence. Quantification of mRNA was enabled by integrating QPA with a magnetic bead-based extraction method using an mRNA–QPA interface reagent. The assay was found to maintain >90% of the maximum signal over a 4 °C range of operational temperatures (64–68 °C). QPA had a dynamic range spanning four orders of magnitude, with a limit of detection of ∼20 pM template molecules using a highly controlled heating and optical system and a limit of detection of ∼250 pM using a less optimal water bath and plate reader. These results demonstrate that this integrated approach has potential as a simple and effective mRNA biomarker extraction and detection assay for use in limited resource settings.

 Please wait while we load your content...
Please wait while we load your content...