Intracellular acid-triggered drug delivery system using mesoporous silica nanoparticles capped with T–Hg2+–T base pairs mediated duplex DNA†
Abstract
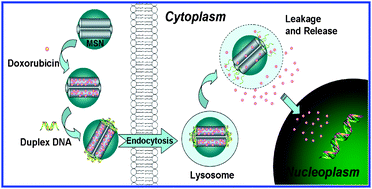
This paper proposed a novel intracellular drug delivery system consisting of mesoporous silica nanoparticles (MSN) functionalized on the pore outlets with an acid-labile DNA molecule-gated switch. In this system, a T–Hg2+–T base pair-mediated double-stranded DNA (dsDNA1) was grafted on the MSN surface as a nanoscopic cap. The delivery system was closed at neutral pH but opened at slightly acidic conditions due to the dissociation of T–Hg2+–T structures and the subsequent melting of dsDNA1. As proof-of-concept, doxorubicin (Dox) was loaded into the dsDNA1-modified MSN (MSN-dsDNA1) as a model drug. Controlled-release studies in water showed that no Dox leaked when the cap was closed and that release occurred immediately after acidification. By alternately changing the pH from 5.0 to 7.2, the DNA cap could be switched “on” and “off”, thereby regulating the partial release of Dox. Further in vitro studies demonstrated that the Dox-loaded MSN-dsDNA1 (MSN-Dox-dsDNA1) could be endocytosed and accumulated within lysosomes, followed by serving as a carrier for the controlled release of Dox into cell nuclei at the lysosomal pH level inside living cells. The cell viability results showed that the inhibitory concentration (IC50) of MSN-Dox-dsDNA1 was low (≈12.5 μg mL−1), while the IC50 of MSN-dsDNA1 (>100 μg mL−1) exceeded eight times higher than that of MSN-Dox-dsDNA1, indicating that MSN-dsDNA1 was fairly biocompatible and indeed served as a drug-carrier for intracellular controlled release. We believe that further developments of this acid-responsive drug-carrier will provide a promising nanodevice for in vivo delivery of therapeutic agents.

 Please wait while we load your content...
Please wait while we load your content...