 Open Access Article
Open Access ArticleIndium-doped SnO2 nanoparticle–graphene nanohybrids: simple one-pot synthesis and their selective detection of NO2†
Shumao
Cui
a,
Zhenhai
Wen
a,
Eric C.
Mattson
b,
Shun
Mao
a,
Jingbo
Chang
a,
Michael
Weinert
b,
Carol J.
Hirschmugl
b,
Marija
Gajdardziska-Josifovska
b and
Junhong
Chen
*a
aDepartment of Mechanical Engineering, University of Wisconsin-Milwaukee, 3200 North Cramer Street, Milwaukee, WI 53211, USA. E-mail: jhchen@uwm.edu; Fax: +1-414-229-6958; Tel: +1-414-229-2615
bDepartment of Physics and Laboratory for Surface Studies, University of Wisconsin-Milwaukee, 1900 E. Kenwood Blvd, Milwaukee, WI 53211, USA
First published on 13th February 2013
Abstract
We demonstrate novel nanohybrids of indium- and ruthenium-doped SnO2 nanoparticles (NPs) on a reduced graphene oxide (RGO) surface prepared using a simple one-pot method at a relatively low temperature. The size of the doped SnO2 NPs on the RGO is as small as 2–3 nm with uniform distribution. We find that the introduction of dopants facilitates the NP nucleation on graphene oxide. The gas sensing responses of the resulting nanohybrids demonstrate that the addition of indium in SnO2 significantly enhances the sensitivity to NO2 compared with RGO–SnO2. The sensor also shows excellent selectivity to NO2 when other common gases such as NH3, H2, CO and H2S, are present. The sensing mechanism responsible for the superior sensitivity and selectivity of the nanohybrids is also discussed.
Introduction
Nanohybrids have been widely studied for various applications due to their enhanced mechanical, electrical, and optical properties.1–5 Recently, graphene (G) and its chemically modified forms (e.g., graphene oxide) have attracted extensive attention as a nanohybrid building block because of its unique mechanical, physical, and chemical properties.6–11 For example, graphene has high mechanical strength (>1060 GPa), high thermal conductivity (∼3000 W m−1 K−1), high electron mobility (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1), and a high specific surface area (2600 m2 g−1).6–9 Graphene-based nanohybrids have been studied for various applications, including energy storage,12–14 catalysis,15,16 drug delivery,17 and sensors.18,19 Graphene is an especially promising gas sensing material because of its high electron mobility and high specific surface area. Various graphene–nanoparticle nanohybrids, including G–Pd, G–Pt, G–SnO2, and G–ZnO2, have been used in sensing applications.19–22 These nanohybrids are usually synthesized either by in situ nanoparticle (NP) growth on graphene in solution or by directly mixing two previously prepared materials. However, the sensing application of graphene-based nanohybrids is still in its infancy.
000 cm2 V−1 s−1), and a high specific surface area (2600 m2 g−1).6–9 Graphene-based nanohybrids have been studied for various applications, including energy storage,12–14 catalysis,15,16 drug delivery,17 and sensors.18,19 Graphene is an especially promising gas sensing material because of its high electron mobility and high specific surface area. Various graphene–nanoparticle nanohybrids, including G–Pd, G–Pt, G–SnO2, and G–ZnO2, have been used in sensing applications.19–22 These nanohybrids are usually synthesized either by in situ nanoparticle (NP) growth on graphene in solution or by directly mixing two previously prepared materials. However, the sensing application of graphene-based nanohybrids is still in its infancy.
Tin oxide (SnO2) is a semiconducting inorganic material with a wide band gap of 3.62 eV at 298 K, which has been widely used for sensing applications.23–25 To achieve high sensitivity and selectivity, both doping and shrinking the size of the nanocrystals of SnO2 have been used.26,27 It is expected that doping SnO2 can be an efficient way to improve sensing performance for the following reasons: (1) the charge carrier concentration of semiconducting SnO2 can be dramatically increased by dopants,28,29 which may facilitate the electron transfer during the interaction with gases; (2) dopants can constrain the growth of SnO2 crystals, forming very fine NPs in hydrothermal methods,30 which will result in a high surface-to-volume ratio of SnO2 NPs that are conducive for gas sensing applications; (3) a large amount of oxygen vacancies and chemisorbed oxygen species are generated by metal dopants,31 and can lead to enhanced sensing response; and (4) the sensor selectivity toward a specific gas can be greatly improved by carefully choosing the dopant. For example, the selectivity to ethanol was enhanced by doping ceria in SnO2,32 and palladium-doped SnO2 was designed to enhance the sensing selectivity to CH4 and H2.33 However, those materials work only at elevated temperatures.
Nitrogen dioxide is a toxic gas produced by combustion in power plants and automobiles that can harm human health and the environment by causing acid rain.34 It has been recommended that the exposure time to 3 ppm NO2 be no longer than 8 h.35 However, it is still a challenge to develop efficient sensors to selectively detect low concentrations of NO2 at room temperature. Indium (In) and ruthenium (Ru) are commonly used as dopants in SnO2 for sensing applications. They have been demonstrated to not only improve sensing properties toward NO2 but also to lower the operating temperature.36,37
In this work, we report on a one-pot strategy to achieve facile and low-cost fabrication of a unique nanohybrid, in which In-doped SnO2 (IDTO) NPs uniformly distribute on reduced graphene oxide (RGO). We investigated the crystal growth mechanism and found that dopants facilitated the nucleation of SnO2 crystals on graphene oxide (GO) surface. This method also can be used to fabricate SnO2 NPs doped with other elements (e.g., Ru) on RGO, and potentially can be used to synthesize other doped metal oxide (e.g., TiO2) or metal oxide nanocomposites on graphene. The Ru-doped SnO2 NPs–RGO hybrids were also synthesized using the same method, and the control experiments further proved the role of dopants in the NP formation. The enhanced sensing performance of RGO/In-SnO2 (RGO–IDTO) toward NO2 was demonstrated at room temperature, reaching a detection limit as low as 0.3 ppm. Moreover, excellent selectivity was also achieved, as verified by testing several other gases with the same device. The tiny NPs offer a large number of active sites for gas adsorption, and the dopants play a critical role in enhancing sensor performance with RGO acting as a conducting channel.
Experimental
Synthesis
GO was prepared by oxidizing graphite powder (Bay Carbon, SP-1 graphite) under acidic conditions according to the modified Hummers method.38 In a typical process to prepare RGO–IDTO, 8 mg GO was dispersed in 20 ml deionized water, and sonicated for 30 min. Then 0.5 ml InCl3 (0.05 M) aqueous solution and 2.5 ml SnCl4 (0.01 M) were added to the GO dispersion in sequence with magnetic stirring (400 rpm). The mixture was sonicated for 10 min to allow for uniform ion adsorption on the GO surface. After that, 15 ml NaBH4 aqueous solution (30 mg per 10 ml) was added drop-wise into the above solution with stirring. Finally, the entire solution was kept at 50 °C on a hotplate for 1 h. The final product was collected by centrifuge. Ru-doped SnO2 NPs decorated RGO (RGO–RDTO) were also prepared using the same method; 1 ml RuCl3 (0.05 M) was used as the dopant source, and all the other chemicals and procedures were the same as those used for the RGO–IDTO synthesis.Characterization
The samples were characterized using a number of techniques. The crystallographic structure of as-produced nanohybrids was investigated by X-ray diffraction (XRD). Scanning electron microscopy (SEM) was carried out with a Hitachi S-4800 electron microscope at an acceleration voltage of 10 kV. The structure of as-produced nanohybrids was characterized by transmission electron microscopy (TEM) (Hitachi H-9000-NAR). High-resolution TEM (HRTEM) and selected area electron diffraction (SAED) (at an acceleration voltage of 300 kV) were used to characterize the crystal structure of the nanohybrids. Energy dispersive X-ray spectroscopy (EDS Noran Si:Li detector) was used to characterize the elemental composition. The surface chemical composition was characterized by using X-ray photoelectron spectroscopy (XPS) (HP 5950A). Raman spectra were taken using a Raman spectrometer (Renishaw 1000B).Gas sensor fabrication and sensing test
To prepare gas sensors composed of RGO–IDTO nanohybrids, gold interdigitated electrodes with finger width and inter-finger spacing of 2 μm and thickness of 50 nm were fabricated by an e-beam lithography process on a silicon wafer with a top SiO2 layer of about 200 nm. The RGO–IDTO nanohybrids were dispersed in N,N-dimethylformamide (DMF), and then a drop (0.1 μl) of the dispersion was cast onto the gold electrode. Low concentration dispersion was used to avoid overlapping RGO sheets. To purify the sensor and improve the electrical contact between the nanohybrids and the gold electrodes, the sensor devices were annealed in a tube furnace at 200 °C for 1 h before sensing tests.The gas sensing properties were tested in an air-tight chamber with electrical feedthroughs. A constant voltage was applied to the electrode gap bridged by the nanohybrids. Then the target gas with certified concentrations was flowed into the chamber, and the change in the current passing through the nanohybrids was monitored and recorded using a Keithley 2602 source meter (Keithley, Cleveland, OH). A typical sensing test cycle consisted of three sequential steps. First, a dry air flow was introduced into the sensing test chamber to record a baseline. Then, a target gas diluted in air was injected to register sensor signals. Finally, the sensor was recovered in a dry air flow. All the flow rates were controlled at 2 l min−1, and the target gases were diluted in dry air. The sensor sensitivity was defined as S = ΔG/G0, where ΔG is the change in the sensor conductance before and after the gas exposure and G0 is the sensor conductance in dry air. The resistance of RGO–IDTO nanohybrids was ∼2 kΩ (or G0 = 0.0005 S) in dry air before target gas exposure.
Results and discussion
Scheme 1 illustrates the preparation procedure of RGO–IDTO nanohybrids. First, indium ions were introduced into the GO dispersion by adding an InCl3 aqueous solution under magnetic stirring. Then, tin ions were slowly added into the above mixture using SnCl4 aqueous solution as the source. After sonication, a NaBH4 solution was slowly dropped into the solution mixture to reduce GO. The obtained solution was then continuously stirred at 50 °C for 1 h and RGO–IDTO nanohybrids were obtained after centrifuging and washing. | ||
| Scheme 1 Schematic illustration for the preparation process of RGO–IDTO nanohybrids. | ||
The morphology of as-produced RGO–IDTO nanohybrids was first examined by a field-emission SEM. An overview of the RGO–IDTO nanohybrids is shown in Fig. S1,† which clearly indicates that the nanohybrids retain the sheet structure typical for graphene. The nanostructure was further investigated using TEM (Fig. 1a), showing that the IDTO nanostructures are uniformly coated on the RGO sheet. A typical magnified TEM image is shown in Fig. 1b, demonstrating that a thin lacy network of IDTO NPs is uniformly distributed on the surface of graphene. The inset of Fig. 1b is the SAED pattern of RGO–IDTO with well-defined rings composed of two parts. The rings marked with red arcs (from the inside to outside) are indexed to rutile SnO2 (110), (101), (200), (211), and (112) planes. Meanwhile, the rings marked with green arcs (from the inside to outside) are indexed to {100}- and {110}-type reflections of graphene, consistent with graphene and with the known structure of RGO with disordered oxygen functional groups. The IDTO nanocrystals anchored on the RGO sheets were further analyzed using HRTEM. As shown in Fig. 1c, IDTO nanocrystals with clear lattice fringes are observed with sizes of about 2–3 nm. The labeled lattice spacing of 0.335 nm corresponds with the unique (110) plane of rutile SnO2. To examine the elemental composition of NPs, EDS was performed and the result indicates that there is indium in the nanohybrid. Similar results were obtained for RGO–RDTO nanohybrids, as shown in Fig. S2.† For substitutional doping, the difference in ionic radii should be less than the Hume-Rothery limit (15%).39 The ionic sizes of Sn4+, In3+, and Ru4+ are 0.083 nm, 0.081 nm, and 0.076 nm, respectively,40,41 with a maximum difference of 8.4%, which lies within the Hume-Rothery limit. Thus, when In and Ru are doped in SnO2, In3+ and Ru4+ can substitute for Sn4+, forming a uniformly stable solid solution, consistent with previous reports.41,42
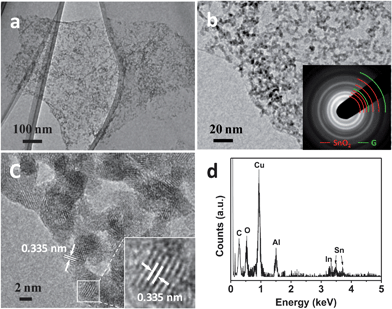 | ||
| Fig. 1 (a and b) TEM images of RGO–IDTO nanohybrids. The inset in image (b) is the SAED pattern of RGO–IDTO. The rings marked with red arcs are indexed to rutile SnO2, and the rings marked with green arc are indexed to graphene. (c) HRTEM image of RGO–IDTO nanohybrids. (d) EDS spectra of RGO–IDTO. Cu and Al are from the sample holder. | ||
To obtain information about the average crystallographic structure, the RGO–IDTO nanohybrid was characterized using XRD (Fig. 2a). For comparison, RGO–SnO2 without dopants was synthesized using a two-step method published previously.43 Briefly, Sn4+ was introduced into the GO dispersion and adsorbed on the surface of GO bonded with oxygen functional groups (e.g., hydroxyl and carbonyl groups) by electrostatic force. Then the precipitate was collected and washed with centrifugation. The product was dried at 80 °C overnight, and RGO–SnO2 was obtained after annealing treatment at 350 °C for 2 h under argon atmosphere. The XRD pattern in Fig. 2a demonstrates the presence of crystalline IDTO NPs through the diffraction peaks corresponding with the (110), (101), (200), and (211) planes of rutile SnO2 (JCPDS 041-1445). The broad peaks indicate that the nanocrystals are tiny, consistent with TEM results. There are no other peaks except for one weak peak around 25.5° corresponding to the (002) plane of few-layer RGO, suggesting that pure IDTO NPs are anchored on the surface of RGO during the synthesis. The XRD pattern of RGO–IDTO is very similar to that of RGO–SnO2, suggesting that there were no phase changes occurring and no nanoscale separation with indium doping in SnO2, which is consistent with the previous report.29 The above results suggest that the dopant ions are homogenously distributed in the SnO2 lattice. The XRD pattern of RGO–RDTO shows similar characteristics (Fig. S3†).
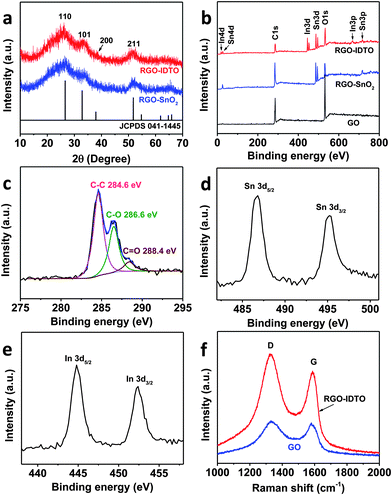 | ||
| Fig. 2 (a) XRD patterns of RGO–SnO2 and RGO–IDTO nanohybrids. (b) XPS spectra of GO, RGO–SnO2, and RGO–IDTO nanohybrids. (c–e) High-resolution XPS spectra of C 1s (c), Sn 3d (d), and In 3d (e) of the RGO–IDTO nanohybrids. (f) Raman spectra of GO and RGO–IDTO nanohybrids. | ||
The surface composition of RGO–IDTO was characterized by XPS. Fig. 2b shows the entire survey spectra of RGO–IDTO, showing the existence of C, O, In, and Sn in the nanohybrid, consistent with the EDS results. The C/O ratios were 1.8 for the initial GO and 1.6 for RGO–IDTO hybrids based on the XPS analysis. Although our electrical measurements presented later suggest that GO has been effectively reduced to RGO during the synthesis process, it is quite challenging to determine the exact degree of reduction of GO because the oxygen signal in the XPS is from both RGO and IDTO. Fig. 2c–e show high-resolution XPS spectra of C 1s, Sn 3d, and In 3d, respectively. The complex C 1s XPS spectra can be fitted to three components with peaks centered at 284.6, 286.6, and 288.4 eV, corresponding with C–C, C–O, and C(O)O, respectively. The binding energies of C–O and C(O)O indicate the existence of oxygen groups in RGO.19Fig. 2d presents the Sn 3d level from IDTO, showing two symmetric peaks due to spin–orbit splitting with binding energies of 495.2 and 486.8 eV for the d3/2 and d5/2 lines, respectively. Similarly, the In 3d level (Fig. 2e) consists of two peaks centered at 452.4 and 444.8 eV for the d3/2 and d5/2 lines, respectively. The as-produced RGO–IDTO nanohybrid was also investigated by Raman spectroscopy (Fig. 2f). The peak at about 1587 cm−1 (G band) corresponds to the in-plane vibration of sp2 carbon–carbon bonds while the peak at about 1330 cm−1 (D band) is attributed to disorders and defects of the graphitic layer.44 The D/G intensity ratio (ID/IG) indicates the extent of π-conjugation and the defect density in the graphitic layer.45 The increase of ID/IG for RGO–IDTO (1.16) compared with that of GO (1.04) suggests a decrease in the average size of sp2 domains and a high concentration of defects, possibly caused by the sonication and reduction process. This increase in ID/IG also agrees with other reported results.46,47
Based on our experiments, we believe that the dopant In plays a critical role in the nucleation of the doped tin oxide. For example, when only Sn and GO (no In) were used in the reaction system with the same experimental procedure, the resulting product consisted of aggregated NPs partially covering the RGO surface (Fig. S4a†). The SAED pattern in Fig. S4b† demonstrates that the NPs on RGO have poor crystalline structure, as evidenced by the broad blurry rings. When using In, the time when In is added is important, affecting the final NP dispersion and crystallization. It was found that similar well-defined RGO–IDTO nanohybrid products were obtained when mixing the two ion sources and then adding them into the GO dispersion, as well as when adding In first followed by adding Sn using the same molar ratio of In/Sn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1, S5a and b†). However, when adding Sn before In, the results were similar to those obtained when adding Sn only into the GO dispersion (Fig. S6†). A possible reason is that Sn adsorbs on the GO surface, occupying most of the available ion adsorption sites on the GO surface. Therefore, limited sites are left for In adsorption, resulting in the poor nanoparticle crystallization and dispersion.
1 (Fig. 1, S5a and b†). However, when adding Sn before In, the results were similar to those obtained when adding Sn only into the GO dispersion (Fig. S6†). A possible reason is that Sn adsorbs on the GO surface, occupying most of the available ion adsorption sites on the GO surface. Therefore, limited sites are left for In adsorption, resulting in the poor nanoparticle crystallization and dispersion.
Both the presence and the amount of dopant ions play a critical role in the formation of IDTO NPs on the GO surface. To investigate the effect of In on the final product, different amounts of In were used in the synthesis of IDTO NPs while keeping the same amount of Sn, i.e., molar ratios of In/Sn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 0.3
1, and 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The as-produced nanohybrids were characterized by TEM and SAED, shown in Fig. S5.† It was found that the samples with the largest amount of In (In/Sn = 1
1. The as-produced nanohybrids were characterized by TEM and SAED, shown in Fig. S5.† It was found that the samples with the largest amount of In (In/Sn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) produced the best crystalline IDTO NPs on the RGO surface (Fig. S5a and b†), as evidenced by the clear nanoparticle distinction and bright sharp SAED rings. The sample synthesized with the smallest amount of In (In/Sn = 0.3
1) produced the best crystalline IDTO NPs on the RGO surface (Fig. S5a and b†), as evidenced by the clear nanoparticle distinction and bright sharp SAED rings. The sample synthesized with the smallest amount of In (In/Sn = 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) produced NPs over the RGO surface with broad diffraction rings (Fig. S5e and f†), indicating poor crystallization of IDTO nanoparticles. Therefore, it is reasonable to conclude that In can lower the nucleation energy of NPs, which means that well-defined IDTO nanocrystals would easily form on the GO surface with a higher concentration of In. We also investigated the function of Ru in the nucleation of RDTO NPs, and found that fine crystalline NPs formed on the RGO when Ru is introduced before adding Sn into the GO dispersion. With the increase of Ru in the solution, a higher density of RDTO nanoparticles formed on the RGO surface (Fig. S7†). Because the dopants encourage the final evenly distributed crystalline NPs on graphene, we propose that the IDTO/RDTO nanocrystals form at positions where dopant ions are located on the GO surface with low nucleation energy at a low temperature.
1) produced NPs over the RGO surface with broad diffraction rings (Fig. S5e and f†), indicating poor crystallization of IDTO nanoparticles. Therefore, it is reasonable to conclude that In can lower the nucleation energy of NPs, which means that well-defined IDTO nanocrystals would easily form on the GO surface with a higher concentration of In. We also investigated the function of Ru in the nucleation of RDTO NPs, and found that fine crystalline NPs formed on the RGO when Ru is introduced before adding Sn into the GO dispersion. With the increase of Ru in the solution, a higher density of RDTO nanoparticles formed on the RGO surface (Fig. S7†). Because the dopants encourage the final evenly distributed crystalline NPs on graphene, we propose that the IDTO/RDTO nanocrystals form at positions where dopant ions are located on the GO surface with low nucleation energy at a low temperature.
Our previous study showed that SnO2 nanocrystals enhance the sensing sensitivity of RGO to NO2.19 To prove dopants can further improve the sensing performance, we investigated the sensing properties of RGO–IDTO to NO2 at room temperature. The dynamic sensing performance of the sensor was measured under different NO2 concentrations (Fig. 3a and b). The electrical conductivity of the hybrid sensor increases upon exposure to NO2, which is consistent with our previous results for RGO–SnO2 sensors. Because NO2 is an oxidizing gas, when NO2 is adsorbed on SnO2 surface, we proposed that there is electron transfer from SnO2 to NO2.4,19 The RGO usually behaves as a p-type semiconductor in air at room temperature and was used as a conducting channel in our device.38 The electron transfer effectively increases the charge carrier (i.e., hole) concentration in RGO, leading to an increase in electrical conductivity. The results also demonstrate that RGO–IDTO nanohybrids show p-type semiconducting behavior, and the semiconducting type of the RGO was not changed after the IDTO NP decoration.
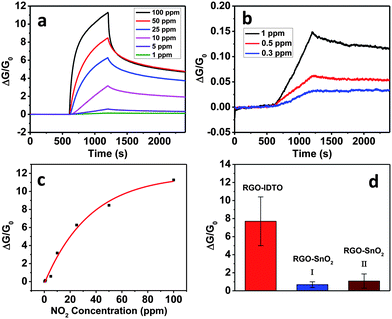 | ||
| Fig. 3 (a and b) Dynamic sensing response of RGO–IDTO toward different NO2 concentrations. (c) Exponential curve of sensitivity as a function of NO2 concentration. (d) Sensitivity comparison of RGO–IDTO and RGO–SnO2 nanohybrids to 100 ppm NO2. | ||
The sensitivity of the sensor decreases upon exposure to lower concentrations of NO2. For the NO2 concentrations investigated (from 0.3 to 100 ppm), the sensing response can be fitted well by an exponential curve, as shown in Fig. 3c. For low concentration detection, the sensor can respond to a concentration level as low as 0.3 ppm, as shown in Fig. 3b, which is an order of magnitude lower than the EPA recommended exposure limit (3 ppm).35 Our sensor thus could be used for room-temperature low concentration NO2 detection. The recovery process of the sensor, however, is slow, taking overnight to completely recover to the initial state. Further sensor optimization is needed to shorten the recovery time.
To prove the sensing enhancement of dopants to NO2, the sensitivities were compared for two types of RGO–SnO2 hybrids fabricated using two different methods. The first series of samples (RGO–SnO2 I) were synthesized using the hydrothermal method described above.43 The second series of samples (RGO–SnO2 II) were synthesized by loading SnO2 NPs on RGO using a mini-arc plasma source.19 The sensitivity of the RGO–IDTO nanohybrids is much higher than that of RGO–SnO2 (Fig. 3d), indicating that In doping in SnO2 NPs can greatly enhance the sensitivity. This result can be attributed to the increase of oxygen species (e.g., Oδ− adsorbates) on the nanoparticle surface by introducing indium as a dopant. As investigated by density functional theory (DFT) calculations, the interaction between the SnO2 surface and NO2 molecules can be described as follows: NO2 (gas) + Oδ− = NO3δ− (adsorption), where NO2 is attached to Oδ− on the SnO2 surface, forming a NO3δ− complex, with electron transfer occurring from the nanoparticle to NO2.48 A higher sensitivity suggests more NO2 molecular adsorption and more electron transfer from the NPs to NO2. Moreover, it has been found that dopants in SnO2 increase the number of oxygen vacancies,31 which in turn can easily dissociate oxygen molecules and thus form chemisorbed oxygen species.49 Since our samples were exposed to air before testing, oxygen in the air might be dissociated and chemisorbed on the IDTO surface. This is also consistent with other observations that the dopant facilitates adsorption of oxygen molecules and formation of oxygen ions on the SnO2 surface.50 The high sensitivity might also be attributed to the tiny size of IDTO nanocrystals due to their large surface-to-volume ratio, which leads to ample adsorption sites in the sensing process and thus an enhanced sensitivity. For NO2 detection, other efficient graphene-based hybrid sensors have also been reported, such as G–WO3 and RGO–Cu2O sensors.51,52 Our RGO–IDTO sensors reported here have a comparable lower detection limit with RGO–Cu2O sensors, which are better than G–WO3 sensors. However, there was no report on the selectivity of those nanohybrids.
In order to probe the selectivity of RGO–IDTO nanohybrid sensors, the same sensor was measured against several other gases, including H2S, CO, H2, and NH3. The sensing test cycle was the same as that for measuring NO2, and the dynamic responses are shown in Fig. 4a. The sensor showed very weak response to all gases except NO2. The device conductance decreased when the sensor was exposed to NH3, indicating electron transfer from NH3 to the nanohybrids. However, the exposure to other gases led to an increase of the conductance, suggesting the electron transfer is in the opposite direction, i.e., from the nanohybrids to the gas molecules. The sensitivity comparison shown in Fig. 4b demonstrates that the response to other testing gases is negligible compared with that of NO2, indicating that our sensor has very good selectivity. Here, we suggest a “superposition effect” as one possible mechanism for the high selectivity: our previous experimental and theoretical studies showed that SnO2 selectively enhanced the sensitivity of MWCNTs or RGO to NO2 at room temperature.19,48 It was also reported that multiple In2O3 nanowire can achieve selective detection of NO2 with other chemical gases such as NH3, O2, CO, and H2.53 Therefore, the differential selectivity of the RGO–IDTO hybrids was maximized by doping indium in SnO2 for this study due to the same gas selectivity enhancement. However, more work is needed to better understand the underlying mechanism.
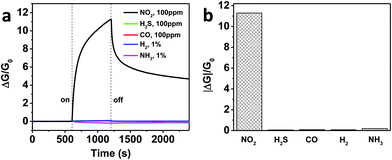 | ||
| Fig. 4 Comparison of sensing responses (a) and sensitivity (b) to various gases. | ||
Conclusions
In summary, RGO–IDTO and RGO–RDTO nanohybrids were successfully synthesized using a simple one-pot aqueous method at low temperature. The morphology characterization results show that In- and Ru-doped SnO2 NPs are evenly distributed on the RGO surface, and that the dopants are successfully incorporated into the SnO2 nanocrystals. The size of the doped nanoparticles is very small, about 2–3 nm. The dopants lower the nucleation energy of the ions on GO and lead to crystalline IDTO or RDTO NPs on the RGO surface. Such nanohybrids are very promising for sensitive and selective detection of NO2. The as-prepared RGO–IDTO showed a much higher sensitivity than RGO–SnO2, indicating the sensing enhancement function of indium doping. The dopants also induced a large number of oxygen vacancies in the nanocrystals, leading to an increase in the number of surface oxygen ion species that can react with NO2 gas molecules. The highly selective sensing to NO2 can be understood as a “superposition effect” of selectivity in the hybrids. This preparation method opens up a simple one-pot approach to synthesize various metal-doped metal oxide nanoparticle–graphene nanohybrids for a wide range of applications such as sensors and catalysis.Acknowledgements
The authors acknowledge financial support from the National Science Foundation (CMMI-0900509), and from the Research Growth Initiative Program of the University of Wisconsin-Milwaukee (UWM). The SEM imaging was conducted at the UWM Bioscience Electron Microscope Facility, and TEM analyses were conducted in the UWM Physics HRTEM Laboratory. Sensor electrodes were fabricated at CNM of Argonne National Laboratory, supported by U.S. DOE (DE-AC02-06CH11357).References
- A. C. Balazs, T. Emrick and T. P. Russell, Science, 2006, 314, 1107 CrossRef CAS.
- L. L. Beecroft and C. K. Ober, Chem. Mater., 1997, 9, 1302 CrossRef CAS.
- H. Y. Fan, K. Yang, D. M. Boye, T. Sigmon, K. J. Malloy, H. F. Xu, G. P. Lopez and C. J. Brinker, Science, 2004, 304, 567 CrossRef CAS.
- G. H. Lu, L. E. Ocola and J. H. Chen, Adv. Mater., 2009, 21, 2487 CrossRef CAS.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9, 1002 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS.
- A. H. Castro Neto, F. Guinea, N. M. R. Peres, K. S. Novoselov and A. K. Geim, Rev. Mod. Phys., 2009, 81, 109 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- D. R. Dreyer, R. S. Ruoff and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 9336 CrossRef CAS.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- S. H. Lee, H. W. Kim, J. O. Hwang, W. J. Lee, J. Kwon, C. W. Bielawski, R. S. Ruoff and S. O. Kim, Angew. Chem., Int. Ed., 2010, 49, 10084 CrossRef CAS.
- D. H. Wang, R. Kou, D. Choi, Z. G. Yang, Z. M. Nie, J. Li, L. V. Saraf, D. H. Hu, J. G. Zhang, G. L. Graff, J. Liu, M. A. Pope and I. A. Aksay, ACS Nano, 2010, 4, 1587 CrossRef CAS.
- A. L. M. Reddy, A. Srivastava, S. R. Gowda, H. Gullapalli, M. Dubey and P. M. Ajayan, ACS Nano, 2010, 4, 6337 CrossRef CAS.
- J. R. Potts, D. R. Dreyer, C. W. Bielawski and R. S. Ruoff, Polymer, 2011, 52, 5 CrossRef CAS.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380 CrossRef CAS.
- I. V. Lightcap, T. H. Kosel and P. V. Kamat, Nano Lett., 2010, 10, 577 CrossRef CAS.
- X. Y. Yang, X. Y. Zhang, Y. F. Ma, Y. Huang, Y. S. Wang and Y. S. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- M. Shafiei, P. G. Spizzirri, R. Arsat, J. Yu, J. du Plessis, S. Dubin, R. B. Kaner, K. Kalantar-Zadeh and W. Wlodarski, J. Phys. Chem. C, 2010, 114, 13796 CAS.
- S. Mao, S. M. Cui, G. H. Lu, K. H. Yu, Z. H. Wen and J. H. Chen, J. Mater. Chem., 2012, 22, 11009 RSC.
- M. Gautam and A. H. Jayatissa, J. Appl. Phys., 2012, 111, 094317 CrossRef.
- J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Adv. Mater., 2010, 22, 4877 CrossRef CAS.
- Q. W. Huang, D. W. Zeng, H. Y. Li and C. S. Xie, Nanoscale, 2012, 4, 5651 RSC.
- M. Batzill and U. Diebold, Prog. Surf. Sci., 2005, 79, 47 CrossRef CAS.
- M. Batzill, Sensors, 2006, 6, 1345 CrossRef CAS.
- Q. H. Wu, J. Li and S. G. Sun, Curr. Nanosci., 2010, 6, 525 CrossRef CAS.
- C. N. Xu, J. Tamaki, N. Miura and N. Yamazoe, Sens. Actuators, B, 1991, 3, 147 CrossRef.
- Y. D. Wang, I. Djerdj, M. Antonietti and B. Smarsly, Small, 2008, 4, 1656 CrossRef CAS.
- D. Fattakhova-Rohfing, T. Brezesinski, J. Rathousky, A. Feldhoff, T. Oekermann, M. Wark and B. Smarsly, Adv. Mater., 2006, 18, 2980 CrossRef.
- Y. D. Wang, T. Brezesinski, M. Antonietti and B. Smarsly, ACS Nano, 2009, 3, 1373 CrossRef CAS.
- M. V. Vaishampayan, R. G. Deshmukh, P. Walke and I. S. Mulla, Mater. Chem. Phys., 2008, 109, 230 CrossRef CAS.
- M. Acciarri, R. Barberini, C. Canevali, M. Mattoni, C. M. Mari, F. Morazzoni, L. Nodari, S. Polizzi, R. Ruffo, U. Russo, M. Sala and R. Scotti, Chem. Mater., 2005, 17, 6167 CrossRef CAS.
- F. Pourfayaz, A. Khodadadi, Y. Mortazavi and S. S. Mohajerzadeh, Sens. Actuators, B, 2005, 108, 172 CrossRef.
- J. K. Choi, I. S. Hwang, S. J. Kim, J. S. Park, S. S. Park, U. Jeong, Y. C. Kang and J. H. Lee, Sens. Actuators, B, 2010, 150, 191 CrossRef.
- J. M. Samet, Inhalation Toxicol., 2007, 19, 1021 CrossRef CAS.
- B. Brunekreef and S. T. Holgate, Lancet, 2002, 360, 1233 CrossRef CAS.
- J. Kaur, R. Kumar and M. C. Bhatnagar, Sens. Actuators, B, 2007, 126, 478 CrossRef.
- N. S. Ramgir, I. S. Mulla and K. P. Vijayamohanan, Sens. Actuators, B, 2005, 107, 708 CrossRef.
- S. Mao, K. H. Yu, S. M. Cui, Z. Bo, G. H. Lu and J. H. Chen, Nanoscale, 2011, 3, 2849 RSC.
- W. Hume-Rothery, The structure of metals and alloys, Institute of Metals, London, 1969 Search PubMed.
- Y. Shigesato, Y. Hayashi and T. Haranoh, Appl. Phys. Lett., 1992, 61, 73 CrossRef CAS.
- N. S. Ramgir, Y. K. Hwang, I. S. Mulla and J. S. Chang, Solid State Sci., 2006, 8, 359 CrossRef CAS.
- P. Nguyen, H. T. Ng, J. Kong, A. M. Cassell, R. Quinn, J. Li, J. Han, M. McNeil and M. Meyyappan, Nano Lett., 2003, 3, 925 CrossRef CAS.
- L. S. Zhang, L. Y. Jiang, H. J. Yan, W. D. Wang, W. Wang, W. G. Song, Y. G. Guo and L. J. Wan, J. Mater. Chem., 2010, 20, 5462 RSC.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud'homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36 CrossRef CAS.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558 CrossRef CAS.
- C. H. Xu, J. Sun and L. Gao, Nanoscale, 2012, 4, 5425 RSC.
- M. Zhang, D. Lei, Z. F. Du, X. M. Yin, L. B. Chen, Q. H. Li, Y. G. Wang and T. H. Wang, J. Mater. Chem., 2011, 21, 1673 RSC.
- S. Cui, H. Pu, E. C. Mattson, G. Lu, S. Mao, M. Weinert, C. J. Hirschmugl, M. Gajdardziska-Josifovska and J. Chen, Nanoscale, 2012, 4, 5887 RSC.
- B. Slater, C. R. A. Catlow, D. E. Williams and A. M. Stoneham, Chem. Commun., 2000, 1235 RSC.
- Q. Wan and T. H. Wang, Chem. Commun., 2005, 3841 RSC.
- S. Srivastava, K. Jain, V. N. Singh, S. Singh, N. Vijayan, N. Dilawar, G. Gupta and T. D. Senguttuvan, Nanotechnology, 2012, 23, 205501 CrossRef.
- S. Deng, V. Tjoa, H. M. Fan, H. R. Tan, D. C. Sayle, M. Olivo, S. Mhaisalkar, J. Wei and C. H. Sow, J. Am. Chem. Soc., 2012, 134, 4905 CrossRef CAS.
- D. H. Zhang, Z. Q. Liu, C. Li, T. Tang, X. L. Liu, S. Han, B. Lei and C. W. Zhou, Nano Lett., 2004, 4, 1919 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ta01673k |
| This journal is © The Royal Society of Chemistry 2013 |
