Complexes of oppositely charged polyelectrolytes and surfactants – recent developments in the field of biologically derived polyelectrolytes
Abstract
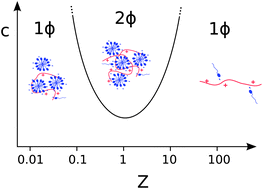
We review the work done on complexes between biopolyelectrolytes such as ionically modified cellulose or
 Please wait while we load your content...
Please wait while we load your content...