Interactive simulations as implicit support for guided-inquiry†
Emily B.
Moore
*a,
Timothy A.
Herzog
b and
Katherine K.
Perkins
a
aUniversity of Colorado Boulder, Department of Physics, Boulder, Colorado 80305, USA. E-mail: emily.moore@colorado.edu
bWeber State University, Department of Chemistry, Ogden, Utah 84408, USA
First published on 2nd April 2013
Abstract
We present the results of a study designed to provide insight into interactive simulation use during guided-inquiry activities in chemistry classes. The PhET Interactive Simulations project at the University of Colorado develops interactive simulations that utilize implicit – rather than explicit – scaffolding to support student learning through exploration and experimentation. In the study, 80 students in a General Chemistry class were given ten minutes to explore the PhET simulation Molecule Polarity in self-selected groups, with no instructions on how to interact with the simulation. Using mouse click data, audio recordings and clicker question responses, we investigated: students' ability to use the simulation by analyzing the extent to which they explored the simulation, the discussions students engaged in during simulation use, and student perceptions of simulation use. We found effective simulation use, with the 22 groups exploring an average of 18 of the 23 available features in Molecule Polarity. Sixty-four percent of student utterances were part of on-topic (polarity) discussion segments, with most off-topic discussions being intermittent and brief. Students largely found the simulation useful for their learning and experienced either brief or no frustration during sim exploration. These results indicate that students in large classes can use interactive simulations designed with implicit scaffolding through exploration, and can do so without frustration overwhelming the perception of value brought by the simulation use. This work suggests that implicitly scaffolded interactive simulations can provide environments that support guided-inquiry learning and channel students into productive inquiry while minimizing the need for explicit guidance.
Introduction
There is increasing development and use of simulations in chemistry classrooms (Kozma et al., 1997; Abraham et al., 2001; Stieff and Wilensky, 2003; Xie and Tinker, 2006; Plass et al., 2012). Previous work has shown the importance of simulation design. For example, the presence of text and audio, as well as the location and presence of different types of representations affect student interpretation and use of simulations (Clark and Mayer, 2007; Stieff et al., 2011; Rodrigues, 2012; Rosenthal and Sanger, 2012). How simulations are used in the classroom also plays an important role in its effectiveness. In particular, the amount and style of guidance provided by the instructor and supporting materials are key factors in how simulations are used and perceived by students (Akaygun and Jones, 2013; Rutten et al., 2012).In inquiry-based learning, students engage in the processes of sense making, discussing ideas, developing evidence-based explanations, and communicating ideas rather than solely being told the content. Substantial research indicates that inquiry learning is an effective approach to learning science (Hmelo-Silver et al., 2007; Furtak et al., 2012). In chemistry, guided-inquiry techniques, including Process Oriented Guided-Inquiry Learning (POGIL) (2012) and Peer-Lead Team Learning (PLTL) (2012), are gaining popularity (Farrell et al., 1999; Lewis and Lewis, 2005; Schroeder and Greenbowe, 2008; Quitadamo et al., 2009; Lewis, 2011; Hein, 2012). With guided-inquiry, students are provided specifically designed materials, and experienced and/or trained facilitators guide them through productive inquiry processes.
Evidence suggests that inquiry learning can be supported by the use of computer simulations (Lee et al., 2010; Smetana and Bell, 2011; Rutten et al., 2012). Simulations can provide students with the opportunity to: interact with dynamic visualizations, allowing for focused exploratory inquiry; engage in rapid feedback cycles, making cause and effect relationships readily apparent; and utilize multiple representations, linking macroscopic, microscopic and/or symbolic representations around a single concept. In this work, we investigate how students use and perceive use of an interactive simulation – designed with implicit scaffolding – in a large chemistry class when given no explicit use instructions.
The simulation used in this work is part of the PhET Interactive Simulations suite, which includes a growing list of over 30 chemistry specific simulations, available for free at http://phet.colorado.edu (Lancaster et al., 2013, in press). PhET interactive simulations (sims) are unique in that they are designed specifically to support inquiry with minimal explicit guidance through the use of implicit scaffolding. Implicit scaffolding is scaffolding built into the design elements and interactivity of PhET sims – resulting in students being guided without feeling guided (Paul et al., 2012). Implicit scaffolding shifts the source of guidance from explicit, such as a set of written instructions, to implicit, where the guidance is in the form of affordances and constraints designed into the sim (Gibson, 1977; Norman, 1988; Podolefsky et al., unpublished work). The design, location, accessibility, presence (or absence) and interactivity of each representation and tool in the sims are carefully selected to guide students into productive inquiry without the need for additional text or auditory guidance within the sim. Each sim is student tested extensively during the design process, ensuring that its design is intuitive and supports student inquiry without requiring explicit guidance from sim use instructions. This design approach allows for flexible use by enabling support through a range of external guidance styles – from none or minimally guided to highly guided.
This work details a baseline study of the interaction between students and a PhET interactive sim in a large classroom context – without explicit guidance from a written activity sheet or from instructor facilitation. From this data we can determine in a classroom context how students use the sim, perceive use of the sim and where explicit guidance may be beneficial – in the form of written questions to guide exploration and instructor facilitation. To accomplish this task, we address the following questions:
– Can students use the sim without instructions?
– Does the sim support content discussion?
– Do students perceive sim interaction as easy and useful, or frustrating and unproductive?
Methods
Data was collected from a single class period on the topics of molecule geometry and polarity. Students were organized into self-selected groups, ranging from 1–4 students each, with most groups consisting of 3 students. Data consisted of video, observation notes, mouse click data and audio recordings from the 25 student groups, and student responses to clicker questions on ‘ease-of-use’, ‘frustration’ and ‘usefulness of the sim for learning’.Class description
Data was collected from a class session in a General Chemistry I course at a public university serving 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 students in the western United States. The General Chemistry I course is the first of a two-semester introduction to chemistry sequence required for science majors. Students were expected to have either completed a chemistry course in high school or taken a college chemistry preparatory course prior to enrolling in General Chemistry I. The class met four times per week for 50 minutes. There were 89 students enrolled in the course. On the date of data collection, there were 80 students in attendance, based on the number of clicker question responses recorded. The course was taught utilizing POGIL-based activities, where students worked in groups to complete written activities, supplemented with the use of clicker questions, instructor mini-lectures and online homework assignments. Prior to this study, the course had covered the topics of nomenclature, stoichiometry, chemical bonding, thermodynamics and periodic trends. The course had utilized the following sims during in-class activities: States of Matter; Build an Atom; Reactants, Products and Leftovers; and Molecule Shapes. Each group was expected to bring at least one personal laptop to class during activities involving sims.
000 students in the western United States. The General Chemistry I course is the first of a two-semester introduction to chemistry sequence required for science majors. Students were expected to have either completed a chemistry course in high school or taken a college chemistry preparatory course prior to enrolling in General Chemistry I. The class met four times per week for 50 minutes. There were 89 students enrolled in the course. On the date of data collection, there were 80 students in attendance, based on the number of clicker question responses recorded. The course was taught utilizing POGIL-based activities, where students worked in groups to complete written activities, supplemented with the use of clicker questions, instructor mini-lectures and online homework assignments. Prior to this study, the course had covered the topics of nomenclature, stoichiometry, chemical bonding, thermodynamics and periodic trends. The course had utilized the following sims during in-class activities: States of Matter; Build an Atom; Reactants, Products and Leftovers; and Molecule Shapes. Each group was expected to bring at least one personal laptop to class during activities involving sims.
Sim description
The Molecule Polarity sim was designed to address the following learning goals for students: predict bond polarity using electronegativity values, indicate polarity with an arrow or partial charge symbols, rank bonds in order of polarity and predict molecular polarity using bond polarity and molecule shape. To target these goals, the sim was designed with three tabs (Fig. 1).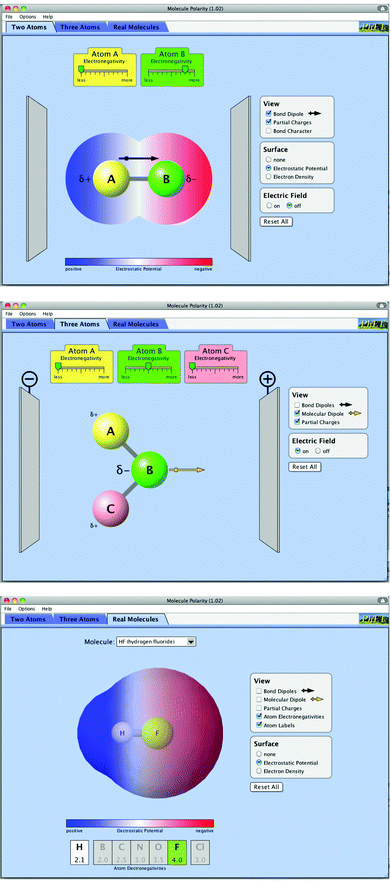 | ||
| Fig. 1 Two Atoms (upper), Three Atoms (middle) and Real Molecule (lower) tabs of the Molecule Polarity sim. | ||
Through interaction with the electronegativity of the molecule's atoms, the Molecule Polarity sim allows for students to connect the symbols used by chemists to denote polarity with the physical meaning of electronegativity. For example, connecting large bond dipoles to large differences in electronegativity.
Sim data
For 22 student groups, we collected the features – tools and representations – students clicked on and the time of the clicks. Note that students were not given explicit instructions on how to interact with the sim, e.g., students were not instructed to click on specific features. Students' use of the sim involves many individual interactions, including repeating actions to make comparisons. For example, in the Two Atoms tab, students tend to move the electronegativity sliders from “less” to “more” and back many times, while observing the effect of this change on the bond dipole. In one group (highlighted in the transcript example in the Results section), the students clicked a total of 74 times during the exploration time, and used a total of 18 features. In this analysis, we focus on the use of features for the first time, to determine the range of exploration with respect to the available features. For example, changing the electronegativity of Atom A for the first time would count as a use of that electronegativity slider on that tab. Further uses of the same feature on the same tab are not included in the analysis below. Collection of this data required students have the wireless Internet enabled on their computer at the start of sim use. Sim use for three student groups was not collected, likely due to a lack of Internet connection.| Feature usea (%) | |
|---|---|
| 22 groups in total | |
| a % of groups that used feature at least once during exploration time. | |
| Two Atoms tab: 8 total features | |
| Atom A electronegativity slider | 95 |
| Atom B electronegativity slider | 95 |
| Molecule rotation | 68 |
| Partial charges checkbox | 91 |
| Bond character checkbox | 82 |
| Electrostatic potential radio button | 95 |
| Electron density radio button | 86 |
| Electric field radio button | 68 |
| Three Atoms tab: 8 total features | |
| Atom A electronegativity slider | 73 |
| Atom B electronegativity slider | 77 |
| Atom C electronegativity slider | 82 |
| Molecule rotation | 36 |
| ABC-angle change | 36 |
| Bond dipole checkbox | 45 |
| Partial charges checkbox | 55 |
| Electric field radio button | 64 |
| Real Molecules tab: 7 total features | |
| Dropdown menu of molecules | 95 |
| Bond dipole checkbox | 100 |
| Molecular dipole | 100 |
| Partial charges checkbox | 100 |
| Atom electronegativities checkbox | 95 |
| Electrostatic potential radio button | 100 |
| Electron density radio button | 100 |
In the Two Atom tab we consider a total of 8 features. Students are able to change the electronegativity of atoms A and B from “less” to “more” using sliders, as well as rotate the diatomic molecule. Students can view the bond character (a scale from “more ionic” to “more covalent”) and partial charges of the atoms. They can also choose to view either the electron density or electrostatic potential surfaces and turn on an electric field (with which the diatomic will align). The Three Atoms tab contains similar features, with the ability to change the electronegativity of a third atom using a slider and change the bond angle of the triatomic by dragging any of the atoms. This design results in a total of 8 features in the Three Atoms tab.
The Real Molecules tab contains a dropdown box, from which students can select different molecules to view inside an embedded Jmol (2012) window. Students can add to the view the bond dipoles, molecular dipole, partial charges and electronegativities, as well as view the electrostatic potential and electron density surfaces. This design results in a total of 7 features in the Real Molecules tab, outside of the Jmol window. We did not count the “Reset All”, “File”, “Options” or “Help” features for any tab or the interactions available inside the Real Molecules Jmol window.
Screencapture data
Seven computers, supplied by the instructor and researcher, were equipped with the screencapture software, Camtasia (2012). This software recorded the computer screen during students' use, while simultaneously recording audio. This screen capture data was used to provide the details of student interaction with the sim during student discussion for the transcript presented in the Results section.Audio data
Each student group was given a digital audio recorder. Audio recordings of the exploration portion of the class from a total of 23 groups were transcribed. The remaining two ‘groups’ consisted of single students who did not talk during their sim use.Because students were typically using the sim while discussing, which sometimes resulted in pauses in verbalizations, audio data was analyzed for topics of discussion rather than episodes of continuous discussion. These topics were consolidated into the following topic categories: ‘group arrangement’ (typically prior to opening the sim), ‘polarity’, ‘polarity with instructor’, ‘school’ or ‘other’. Table 2 shows example transcript excerpts of student discussion from each of the five topic categories.
| Topic | Example transcript | Number of utterances |
|---|---|---|
|
Group arrangement (typically prior to sim use):
Where to put the computer, who is ‘clicking’, etc. |
S1: We did a lot of clicking yesterday. If you would like to do some clicking—that didn't last very long. S1: I think I'm gonna scootch over, make it easier. S1: Ok. | 3 |
|
Polarity:
Sim, polarity & related topics |
S1: Now I'm not sure what the electric field is showing. Oh, there we go. S2: Oh. S1: OK. So it is showing its attraction. OK. S2: Yeah, so this one's more attracted to the positive, this one's more attracted… OK, that makes sense. | 4 |
|
Polarity with Instructor:
Polarity-related discussion between instructor & student |
I: Uh-huh. So this molecular dipole, that's the overall, like, in balance, in charge present in that molecule. S: OK. So since this is fluorine, it's gonna pull that way all the time, pretty much? I: Yup. S: Yup. | 4 |
|
School:
Homework, Lab, etc. (Includes non-chemistry courses) |
S1: What tutor do you have? S2: I have [name], same one, right? S1: All three of us, right? You got [name]? S2: Really? No way. That's awesome. | 4 |
|
Other:
Discussion not classified above |
S1: I didn't work yesterday morning. I usually work at 5. I slept until 10. I cleaned my room. I found $10 cleaning my room. S2: Nice. S1: Did all my laundry. It was really nice. | 3 |
Each transcript was subdivided into segments of discussion on a single topic. Each segment had to contain utterances – individual continuous verbalizations – from at least two students. For example, this exchange – S1: ‘Cause that's more electronegative? S2: Mm-hmm. – consists of two utterances on the topic of ‘polarity’. For a more detailed example of coding and the coding rubric, see the ESI† materials. The authors developed the coding rubric; two researchers – one had not observed the data collection and had not participated in developing the coding rubric – completed the coding independently. The agreement between the coding of the 1832 total utterances into discussion segments was 91%. The agreement between the coding of each of the 141 total discussion segments into the topic categories was 94%.
Clicker response data
Immediately after exploration time, individual student responses to a series of questions were collected using personal response devices, i.e. clickers. The questions probed: sim ‘ease-of-use’, ‘frustration’ and ‘usefulness of the sim for learning.’Results and discussion
Sim use
As an indicator of effective sim use, we present results on how many sim features – tools and representations – the students used during the ten minutes of exploration time. Fig. 2 shows the students' new use of features, in five second increments, during the ten minutes of exploration time. Each line in Fig. 2 corresponds to one group's use of new features over time for that tab, each circle indicates the use of a new feature. Students began interacting with the Two Atoms tab (Fig. 2, top panel), indicated by the heavy new use of features for this tab from 0–3 minutes. After using most or all of the tab's eight features, students moved on to the Three Atoms (Fig. 2, middle panel) and Real Molecules tabs (Fig. 2, lower panel). This tendency results in a cluster of interactions at the beginning of the exploration time (0–3 minutes) for the Two Atoms tab, while the Three Atoms and Real Molecules tabs have group use spread out over minutes 2 through 9. From audio analysis (detailed in the next section) we find that students began interacting with the sim immediately once it opened on their computer screen. The offsets in first feature use for some groups in Fig. 2 are due to the variation in sim access rather than a delay in interaction – some students had the sim already downloaded to their desktop and available for opening once prompted, while others had to be given the USB flash drive and download the sim before opening it.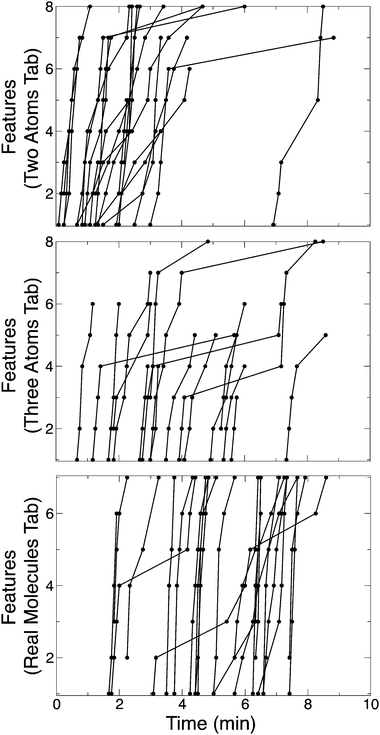 | ||
| Fig. 2 New feature use in the Two Atoms, Three Atoms and Real Molecules tabs. Each line corresponds to one group's use of new features on that tab, circles indicate new feature use. | ||
The average number of new features – out of 23 available – students interacted with during the ten minutes of exploration time is shown in Table 3. Students used the most features in the Two Atoms (6.8 out of 8 features) and the Real Molecules tabs (6.9 out of 7 features). The Three Atoms tab has similar features to the Two Atoms tab, which may explain the lower new feature use for the Three Atoms tab (4.6 out of 8 features). Students may have explored the Two Atoms tab to the extent they felt necessary and then only used the features of the Three Atoms tab that seemed relevant to the students' inquiry. Of the eight student groups that explored four or less features of the Three Atoms tab, five groups had fully explored the Two Atoms tab and the remaining three groups had explored at least five features in the Two Atoms tab. Four groups did not explore the Three Atoms tab at all, though these same groups explored the Two Atoms tab and Real Molecules tab fully. These four groups may have ‘jumped ahead’ to investigate the Real Molecules tab, and then run out of exploration time before being naturally inclined to ‘go back’ to investigate the Three Atoms tab.
While groups used the majority of features at least once during exploration time, a few features were unexplored by many groups (Table 2). One of the most commonly unexplored features of the Three Atoms tab was the ABC-Angle Change – dragging of an atom to change the bond angle. This feature is particularly useful for exploring molecular dipole. Only five of the groups (36%) explored this feature. This suggests that a guided-inquiry activity question – e.g., “How does the ABC-bond angle effect molecule polarity?” – or a prompt – e.g., “Create a rule for predicting how a change of the ABC-bond angle will effect the molecule polarity.” – would be beneficial in guiding students to explore this productive sim interaction.
During the ten minutes of exploration with the Molecule Polarity sim, students interacted with the sim and explored most or all of the available features. This finding indicates that the design of the sim is intuitive for students – supporting the exploration of features – even in a large classroom context where groups did not have access to written instructions or an instructor for guidance. Analysis of the use of new features also provides insight into how students used the sim – sequentially from the first tab to the last tab. This usage pattern suggests that an accompanying guided-inquiry activity might best support the inquiry process promoted through sim design by encouraging exploration of the sim without specific use instructions (which this study suggests may not be necessary) and by posing conceptual questions in a sequence from the first tab to the last before asking students to refer to tabs out of order. An example annotated guided-inquiry activity is provided in the ESI materials.†
Student discussion
Here we analyze the student discussion data during the ten minutes of exploration time, providing insight into the types of discussion the sim use supported. Each group was given an audio recorder, allowing analysis of all group discussions. First we present a single transcript, qualitatively highlighting some of the ways the sim supported discussion on the topic of ‘polarity’. We then present a quantitative summary of all the transcripts, focusing on the range of topics discussed and the amount of discussion on the topic of ‘polarity’ that occurred during sim exploration.This transcript was selected as representative of student exploration with minimal ‘school’ or ‘other’ topic discussion. It was recorded using screen capture software, allowing for detailed descriptions of sim use synced with student utterances. This group consisted of two male students. We present the full transcript from the time the sim opened on the students' computer screen until the point the instructor ends the exploration time. The only utterances not included in the transcript occurred prior to the students opening the sim: four utterances referring to a chemistry lab assignment. These two students were originally part of a larger group that spanned two seating levels in the classroom, but were given a computer and asked to form their own group about two minutes into the exploration time. They had not seen the Molecule Polarity sim in their previous group configuration, as they were on the lower level from the student with the computer. During exploration time, Student 1 (S1) controlled the interaction with the computer. Time is indicated in the (min:sec) format, with respect to the start of exploration time.
| 2:21 | Molecule Polarity opens to the Two Atoms tab. |
| 2:23 | S1: OK. So. OK. So let's see here. [increases Atom B electronegativity to maximum] I'm just messin' around. |
| 2:42 | S2: I think that's what we're supposed to do right now. [increases to maximum then decreases to minimum Atom A electronegativity] |
| 2:50 | S1: OK. So if electron, err, if atom A is more electronegative [increases Atom A electronegativity]—what does this mean? |
| 2:59 | S2: That's the— |
| 3:00 | S1: That means it [the bond dipole arrow] gets smaller? [moves Atom A electronegativity higher, then lower] |
| 3:01 | S2: Yeah. |
| 3:06 | S1: OK, and the same thing here. [moves Atom B electronegativity slider higher, then lower] OK, so the less electronegative that is and the more that is, the farther apart they're gonna be. And if you bring ‘em [electronegativity sliders for Atom A and Atom B] closer together [moves Atom B electronegativity lower and Atom A electronegativity higher]—OK. [moves Atom A electronegativity slider higher and lower] Oh, it switches [direction of bond dipole arrow]. ‘Cause that'd be more [Atom A's electronegativity] and that'd be less [Atom B's electronegativity]. [selects “Partial Charges”, then moves Atom A electronegativity to minimum and Atom B electronegativity to maximum] |
| 3:39 | S1: Makes sense, all right. |
| 3:40 | S2: Yeah. |
Notice how the students were sense making through interaction with the sim, rather than observing some behavior and then sense making. When using the sim, students are in control of the feedback they get; they can pause in their interaction, redo a particular interaction, extend an interaction by going to larger extremes and explore other features, as their inquiry requires. We think that this direct interactivity with the features in the sim supports sense making with the representations, not just about the representations.
S1 started this sim use by changing one electronegativity slider slightly, then exploring the range of the second electronegativity slider. Then he compared the behavior over a range of electronegativity values for each atom. S1 seemed surprised upon observing that his changing of one atom's electronegativity resulted in the bond dipole changing direction, but was quickly able to make sense of this behavior by noting that this interaction had resulted in switching which atom had the higher and lower electronegativity. Next, the students related this effect of the atom electronegativity to what they had learned previously in chemistry class about the periodic table.
| 3:41 | S1: Is this done with the periodic table? Like, why that [partial charge] would be positive and that [partial charge] would be negative? |
| 3:48 | S2: Yeah. |
| 3:49 | S1: ‘Cause that's more electronegative? |
| 3:51 | S2: Mm-hmm. |
| 3:53 | S1: OK. And doesn't—electronegativity goes like that right? |
| 3:59 | S2: Yeah. |
| [selects “Bond Character”, moves Atom A electronegativity from ‘less’ to ‘more’ slowly, sim shows change in bond character from ionic to covalent] | |
| 4:00 | S1: OK. Ionic—so what would cause it to be covalent? Does that mean that they would have to be— |
| 4:16 | S2: —the same. |
| 4:17 | S1: —on the same side, right? |
| 4:18 | S2: Of the same atom, I think. The last two O's. |
| 4:22 | S1: OK, which makes sense, ‘cause they're both the same with the negative. So if you were to go here [moves Atom A electronegativity from ‘more’ to the middle] and there [moves Atom B electronegativity from ‘more’ to the middle’], it's still gonna be covalent. OK. [moves Atom B electronegativity from middle to ‘more’, moves Atom A electronegativity from the middle to ‘more’, pauses, and then from ‘more’ to the middle] |
| [Selects “Electrostatic Potential” view] | |
| 4:50 | S1: OK, that makes sense, dealing with the charge. [selects “Electron Density” view, moves Atom A electronegativity from middle to ‘less’] |
| 5:08 | S1: OK, so electron density deals with electronegativity, the more dense it is, it has a higher electronegativity, right? Why is that? I don't know, either. |
| 5:35 | Selects Three Atoms tab. |
| 5:37 | S1: [rotates molecule] Oh, wow. [moves Atom C electronegativity from ‘less’ to middle, pauses, then moves to ‘more’] |
| 5:42 | S1: OK. |
| 5:50 | S1: So what is this [molecular dipole arrow] pointing to? I'm trying to think how this works here. [moves Atom C electronegativity from ‘more’ to ‘less’] More, less—[moves Atom B electronegativity from the middle to ‘less’ then to ‘more’, then back to the middle, moves Atom C electronegativity from ‘more’ to the middle, pauses, then to ‘less’, selects “Partial Charges”, then “Bond Dipole”] |
| 6:26 | S1: OK. So those are just like when we were lookin' at the two [Two Atoms tab]. |
| 6:35 | S2: Yeah. |
| 6:37 | S1: So it's always gonna be pointing towards the negative. And then what does this [molecular dipole] signify? |
| 6:46 | S2: The dipole. I guess that's—I don't know. |
| 6:51 | S1: I don't know how to explain that. |
| 6:53 | S2: Maybe the sum of the two bond dipoles. |
| 6:55 | S1: uh huh. [deselects “Molecular Dipole”] |
Though the students did not explore this concept further on this tab, with the support provided by the sim – the scaffolding provided by the first tab, the juxtaposition of dipole representations, the ability to choose the representations – the students were able to construct an idea, albeit tentative, of what a molecular dipole arrow represented. This idea could be further developed during a guided-inquiry activity, with guidance in the form of a written question supplemented by instructor facilitation.
| 7:04 | Selects Real Molecules tab. |
| 7:09 | S1: HF. |
| 7:14 | S1: So the arrow should be pointing that way, OK? [selects “Bond Dipole”, then “Molecule Dipole”] Same with that. Partial charge, positive–negative, right? [selects “Partial Charges”, deselects, then selects, selects “Atom Electronegativities”] |
| 7:38 | S1: Yep, yeah that makes sense. [selects “Electrostatic Potential” view] |
| 7:45 | S2: OK and that deals with the electronegativity it would be higher and with the density—[selects “Electron Density” view, selects “Reset All”] |
| 7:58 | S1: OK, I know this [selects H2molecule] should be a covalent bond. [selects “Bond Dipole”, then deselects “Bond Dipole”, selects “Molecular Dipole” then deselects “Molecular Dipole”, selects "Partial Charges", then deselects then selects “Partial Charges” again] |
| 8:47 | S1: So it's all the same, [selects “Molecular Dipole”, then “Bond Dipole”] which makes sense. [Turns “Atom Electronegativities” on.] |
| 9:31 | S1: All right. [reads] So what factors— |
| Instructor ends exploration time. |
After successfully predicting dipole direction and electronegativity for two molecules, the group pauses sim interaction – possibly listening to a loud discussion in a neighboring group – and then S1 began to read the prompt that had been projected at the front of the room during exploration. The instructor interrupted by prompting students to start answering the clicker questions regarding their sim use experience.
Throughout this sim use, the students interacted with the sim heavily, explored the various tabs and features available, discussed, engaged in sense making and ended by making predictions that utilized knowledge gained during the exploration time. While this group was consistent in their discussion about polarity throughout the exploration time, we observed many other groups engaging in the same style of sim use, with varying amounts of non-polarity discussion mixed in.
We now present results regarding the amount of polarity and non-polarity discussion across all of the student groups in Table 4. Each of the 23 group audio recordings was transcribed, and each transcript was subdivided into discussion segments, based on the topic of discussion – resulting in 141 discussion segments across the 23 student groups. Each of these discussion segments was coded as consisting of one of five topics (Table 2): ‘group arrangement’ (typically prior to sim use), ‘polarity’, ‘polarity with instructor’, ‘school’ and ‘other’. Each transcript contained an average of six discussion segments (ranging from 2–12 segments) and 80 utterances (ranging from 23–143 utterances). Individual utterances ranged from a single interjection up to 80 words in length, consisting of multiple sentences.
| Topic | Discussion segments (%) | Utterances (%) |
|---|---|---|
| 141 in total | 1832 in total | |
| Group arrangement (prior to sim use) | 16 | 6 |
| Polarity | 38 | 62 |
| Polarity with instructor | 4 | 2 |
| School | 15 | 10 |
| Other | 27 | 20 |
While the instructor had not intended to participate in discussions during the exploration time, two groups asked the instructor direct questions, and the instructor responded, resulting in the small number of ‘polarity with instructor’ discussion segments. The majority of the discussions consisted of segments on ‘polarity’ (38%), ‘school’ (15%) and ‘other’ (27%). There was a moderate amount of ‘group arrangement’ discussion segments, virtually all prior to the opening or start of use of the sim. Interestingly, even though ‘school’ and ‘other’ segments made up a combined 42% of the total discussion segments, they only made up a combined 30% of the total utterances. In comparison, chemistry concept discussion made up 38% of the discussion segments, but 62% of the total utterances, more than twice that of the ‘school’ and ‘other’ utterances. Most utterances were made during discussion segments about ‘polarity’, while ‘school’ and ‘other’ discussions segments contained comparatively less utterances. The average number of utterances in ‘polarity’ topics was 21 (ranging from 2–85 utterances per segment), compared to an average of 10 utterances for the ‘other’ segments (ranging from 2–49 utterances per segment). These results are consistent with our general observations that the student groups discussed primarily the topic of polarity with some brief and intermittent non-polarity discussions.
The number of utterances on a specific topic does not necessarily indicate amount of time spent on that topic. For example, in the transcript above, the students were not necessarily verbalizing during the time they were interacting with the sim, even when the sim interactions indicated active sense making about polarity. After first changing from the Two Atoms to the Three Atoms tab (at 5:35), nearly a minute went by during which there were only three utterances (from 5:35–6:26), even though interaction with the sim was consistent and systematic. In contrast, when the students were discussing the relationship between electronegativity and the periodic table (3:41–3:59) less than twenty seconds went by during which there were six utterances. From observations and audio recording analysis it was common across groups that sim interaction resulted in pauses in conversation, suggesting that the amount of time spent on the topic of polarity was even greater than would be inferred based solely on the number of conversation segments or utterances.
Clicker question results
With the sim data and audio recorders, we were able to capture data that indicated what sim features students interacted with and what the students discussed. Student resistance to innovation in the classroom has been reported in the literature (Phelps, 1996; Hein, 2012), so we also wanted to know if students found the experience of using the sim with minimal instruction easy and useful, or frustrating and useless. Here, we present data on student perceptions of sim use, based on responses to clicker questions asked immediately after exploration time (Table 5). Most students (70%) indicated that the sim was ‘easy’ or ‘very easy’ to use. The remaining students responded ‘neutral’, with no students responding that the sim was difficult to use. This is consistent with observations of the class during the exploration time and analysis of the audio data; students were predominately using the sim and discussing the sim topic, not discussing how to use the sim.| Category | Question/prompt | Multiple choice options | Response (%) |
|---|---|---|---|
| Ease-of-use (N = 80) | The molecule polarity simulation was… | Very easy to use | 29 |
| Easy to use | 41 | ||
| Neutral | 30 | ||
| Difficult to use | 0 | ||
| Very difficult to use | 0 | ||
| Usefulness for learning (N = 76) | How useful do you think the simulation was or will be for your learning? | Very useful | 8 |
| Useful | 37 | ||
| Somewhat useful | 47 | ||
| Mostly useless | 7 | ||
| Completely useless | 1 | ||
| Frustration (N = 80) | While playing with the molecule polarity simulation, did you feel frustrated? | No | 89 |
| Yes | 11 | ||
| Frustration description (N = 79) | Which best describes any frustration you might have had while using the Molecule Polarity simulation: | I was not frustrated at all. | 57 |
| I was very briefly frustrated. | 33 | ||
| I was frustrated for a short amount of time. | 8 | ||
| I was frustrated for a significant amount of time. | 3 | ||
| I was frustrated for more than half the time. | 0 | ||
Regarding usefulness for learning and frustration, a large majority of students (92%) responded that the sim would be ‘somewhat’ to ‘very’ useful for their learning, and when asked if they felt frustrated during sim use, only 11% of students responded “Yes”. When asked to describe any frustration with the sim, 57% of students responded that they had no frustration, with 33% responding that they had experienced ‘very brief’ frustration. The ‘no frustration’ and ‘very brief frustration’ responders made up 96% of students that responded “No” to the previous yes/no frustration question. Only 3% of students indicated feeling frustrated ‘for a significant amount of time’.
Possible study limitations
Prior to this study, students had used four PhET sims with in-class activities. Student experience with the Molecule Polarity sim could have been affected by these previous uses of PhET sims, making the results we present dependent on prior experience with PhET sims. We feel this does not diminish the results, as informal feedback from PhET users suggests that it is common for instructors to use more than one sim during a course.It is also possible that the explicit presence of audio recorders could have lead students to engage differently with the sim than they would have without the presence of audio recorders. While it is impossible to know exactly what affect the audio recorders had on student behavior, instructor and observing researcher noted that student behavior appeared consistent with previous observations of classroom sim use without audio recorders. As described in the Student Discussion Section, a range of student discussion topics were recorded, suggesting that students felt comfortable having both on-topic and off-topic discussions.
The role of implicit scaffolding
We posit that the implicit scaffolding designed into the sim supported students' use, discussion and perceptions through multiple mechanisms. To highlight examples of what we consider to be support provided by implicit scaffolding, we focus on a specific case – student's use of the Three Atoms tab shown in the transcript above (minutes 2:21–6:55). We believe that the sim's Two Atoms tab implicitly scaffolded the students' productive interactions with the Three Atoms tab – concluding that the molecular dipole is the sum of the bond dipoles – through: (1) consistent design, (2) perceived ‘challenges’ and (3) location of features across the two tabs.The consistency across design features and layout between the Two Atoms and Three Atoms tab could serve to minimize cognitive load. On the Two Atoms tab, students interacted with many of the same features available on the Three Atoms tab. Thus, when switching to the Three Atoms tab, students would have been familiar with many of the features, allowing increased cognitive attention for sense making. In addition, this consistency in design could also serve to cue students to look for and focus on what features are new.
The sim was also designed to implicitly scaffold students to attend to increasingly difficult – though related – challenges, through the selection of available representations. The Two Atoms tab was designed specifically to implicitly scaffold students towards an understanding of bond dipoles, a component of the concept of molecular dipole. The students in the transcript first attended to the ‘challenge’ of making sense of the bond dipole with the sim, indicated by the effect of their first action with the Two Atoms tab (moving the atom electronegativity sliders): a noticeable change in the bond dipole arrow representation. Upon moving to the Three Atoms tab, students encountered a similar scenario, interaction with the atom electronegativity sliders effected an arrow representation – bond dipole, but in this Three Atoms tab the effected arrow is the molecular dipole. The students perceived the ‘challenge’ to complete while on the Three Atoms tab was to make sense of this new arrow representation. This perception of ‘challenge’ serves affective as well as conceptual goals. The students not only perceived a specific conceptual goal while using the sim, but successful completion of a perceived ‘challenge’ provides a positive sense making experience for the students. This positive experience could serve to increase students' intrinsic motivation to seek out other conceptual ‘challenges’. In the example transcript, the students' successful completion of the ‘challenge’ of making sense of the bond dipole arrow (indicated by utterances at 3:39 and 3:40) could have contributed to their motivation to persist through the ‘challenge’ of sense making with the molecular dipole arrow.
The location of tools in the Three Atoms tab could also have played a key role in implicitly scaffolding students sense making. To make sense of the molecular polarity arrow, the students utilized the ability to juxtapose the “Molecular Dipole” arrow with the “Bond Dipole” arrow they had previously explored successfully. The “Bond Dipole” checkbox was intentionally located just below the “Molecular Dipole” checkbox, because it is a conceptually related representation and the location near the top of the toolbox is where students typically start tool exploration (based on student interviews during sim development). The location made the “Bond Dipole” checkbox readily accessible when students began looking for tools to assist their sense making process.
Thus, while PhET sims avoid embedded directions, they provide significant scaffolding for students' interactions, through the design.
Conclusion
In this study, students were given ten minutes of exploration time with a PhET interactive sim – without explicit instructions on sim use. During this exploration time, students interacted with the majority of features available and engaged in chemistry content (molecule polarity) discussions with intermittent ‘school’ or ‘other’ discussions. After the exploration time, the majority of students indicated that use of the sim was easy, productive for their learning, and occurred without frustration.Data on sim feature use indicates that students in large lecture classes can use implicitly scaffolded sims without explicit instructions on how to use the sim. From analysis of audio transcripts, we found that students engaged in content-rich discussions while being supported by the implicitly scaffolded interactive sim. This result suggests that it is possible to have effective guided-inquiry group work in a large lecture setting while minimizing the need for explicit instructions. This finding opens up opportunities, as well as further questions, about the possible roles interactive simulations can play in a guided-inquiry curriculum.
These findings suggest several promising avenues for further research with the potential to lead to new classroom innovations, such as detailed investigations of the mechanisms through which implicit scaffolding can support productive inquiry, and ways to effectively couple and facilitate guided-inquiry activities with interactive simulations. With less explicit instructions, students could feel more autonomous and more competent while engaging in productive guided-inquiry with the supports provided by an interactive simulation. This change in students' role could also result in increased interest in chemistry by students. Use of the supports provided by the PhET sims might also allow students the opportunity to experience self-directed exploration and development of science concepts in a classroom, which may shift their epistemological framing of the classroom experience towards learning as actively engaging rather than passively participating or following directions. (Bing and Redish, 2012)
Acknowledgements
The authors would like to acknowledge the contributions of Sam Reid for sim data collection and analysis, Jesse Garrison for his assistance in analysis of the audio transcripts and the development team for the Molecule Polarity interactive simulation, with design lead by Kelly Lancaster and software development by Chris Malley. This work was funded by the National Science Foundation (CCLI-0817582).References
- Abraham M. R., Gelder J. I. and Haines K., (2001), A web-based molecular-level inquiry laboratory activity, Chem. Educ., 6, 307–308.
- Akaygun S. and Jones L. L., (2013), How does level of guidance affect understanding when students use a dynamic simulation of liquid–vapor equilibrium? in Devetak I. and Glazer S. A. (ed.), Active Learning and Understanding in the Chemistry Classroom, Springer, in press.
- Bing T. J. and Redish E. F., (2012), Epistemic complexity and the journeyman-expert transition, Phys. Rev. ST Phys. Educ. Res., 8, 010105.
- Camtasia Studio: Screen Recording and Editing Software, (2012), Retrieved November 9, 2012, from http://www.techsmith.com/camtasia.html.
- Clark R. C. and Mayer R. E., (2007), in Taff R. (ed.), e-Learning and the science of instruction: proven guidelines for consumers and designers of multimedia learning, 3rd edn, San Francisco, CA: Pfeiffer.
- Farrell J. J., Moog R. S. and Spencer J. N., (1999), A guided-inquiry general chemistry course, J. Chem. Educ., 76, 570–574.
- Furtak E. M., Seidel T., Iverson H. and Briggs D. C., (2012), Experimental and quasi-experimental studies of inquiry-based science teaching: a meta-analysis, Rev. Educ. Res., 82, 300–329.
- Gibson J., (1977), The theory of affordances, in Shaw R. and Bransford J. (ed.), in Perceiving, Acting, and Knowing: Toward an Ecological Psychology, Hillsdale, NJ: Lawrence Erlbaum, pp. 67–82.
- Hein S. M., (2012), Positive impacts using POGIL in organic chemistry, J. Chem. Educ., 89, 860–864.
- Hmelo-Silver C. E., Duncan R. G. and Clark A. C., (2007), Scaffolding and achievement in problem-based and inquiry learning: a response to Kirschner, Sweller, and Clark (2006), Educ. Psychol., 42, 99–107.
- Jmol: an open-source Java viewer for chemical structures in 3D, (2012), Retrieved November 9, 2012, from http://www.jmol.org/.
- Kozma R. B., Russell J. W., Jones T., Wykoff J., Marx N. and Davis J., (1997), Use of simultaneous-synchronized macroscopic, microscopic, and symbolic representations to enhance the teaching and learning of chemical concepts, J. Chem. Educ., 74, 330–334.
- Lancaster K., Moore E. B., Parson R. and Perkins K., (2013), Insights from using PhET's design principles for chemistry simulations, in Suits J. and Sanger M. (ed.), Pedagogic Roles of Animations and Simulations, ACS Symposium Series, in press.
- Lee H.-S., Linn M. C., Varma K. and Liu O. L., (2010), How do technology-enhanced inquiry science units impact classroom learning? J. Res. Sci. Teach., 47, 71–90.
- Lewis S. E., (2011), Retention and reform: an evaluation of peer-led team learning, J. Chem. Educ., 88, 703–707.
- Lewis S. E. and Lewis J. E., (2005), Departing from lectures: an evaluation of a peer-led guided inquiry alternative, J. Chem. Educ., 82, 135–139.
- Norman D., (1988), The design of everyday things, New York: Basic Books.
- Paul A., Podolefsky N. and Perkins K., (2012), Guiding without feeling guided: implicit scaffolding through interactive simulation design, Proceedings of the 2012 Physics Education Research Conference, AIP Press.
- Podolefsky N., Moore E. B. and Perkins K., unpublished work.
- Phelps A. J., (1996), Teaching to enhance problem solving: it's more than the numbers, J. Chem. Educ., 73, 301–304.
- Plass J. L., Milne C., Homer B. D., Schwartz R. N., Hayward E. O., Jordan T., Verkuilen, J., et al., (2012), Investigating the effectiveness of computer simulations for chemistry learning, J. Res. Sci. Teach., 49, 394–419.
- Process Oriented Guided Inquiry Learning, (2012), Retrieved November 9, 2012, from http://www.pogil.org.
- Quitadamo I. J., Brahler C. J. and Crouch G. J., (2009), Peer-led team learning: a prospective method for increasing critical thinking in undergraduate science courses, Science, 18, 29–39.
- Rodrigues S., (2012), Using simulations in science: an exploration of pupil behaviour, in Issues and Challenges in Science Education Research: Moving Forward, Berlin: Springer, pp. 209–223.
- Rosenthal D. P. and Sanger M. J., (2012), Student misinterpretations and misconceptions based on their explanations of two computer animations of varying complexity depicting the same oxidation–reduction reaction, Chem. Educ. Res. Pract., 13, 471–483.
- Rutten N., van Joolingen W. R. and van der Veen J. T., (2012), The learning effects of computer simulations in science education, Comput. Educ., 58, 136–153.
- Schroeder J. D. and Greenbowe T. J., (2008), Implementing POGIL in the lecture and the Science Writing Heuristic in the laboratory—student perceptions and performance in undergraduate organic chemistry, Chem. Educ. Res. Pract., 9, 149–156.
- Smetana L. K. and Bell R. L., (2011), Computer simulations to support science instruction and learning: a critical review of the literature, Int. J. Sci. Educ., 34, 1337–1370.
- Stieff M. and Wilensky U., (2003), Connected chemistry—incorporating interactive simulations into the chemistry classroom, J. Sci. Educ. Technol., 12, 285–302.
- Stieff M., Hegarty M. and Deslongchamps G., (2011), Identifying representational competence with multi-representational displays, Cognition Instruct., 29, 123–145.
- The Center for Peer Lead Team Learning, (2012), Retrieved November 9, 2012, from http://www.pltl.org.
- Xie Q. and Tinker R., (2006), Molecular dynamics simulations of chemical reactions for use in education, J. Chem. Educ., 83, 77–83.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3rp20157k |
| This journal is © The Royal Society of Chemistry 2013 |
