Chemoselective one-pot synthesis of β-keto sulfones from ketones†
Abstract
A practical method to synthesize substituted β-keto sulfones directly from ketones at room temperature has been developed. This method involves the nucleophilic addition of a base generated enolate to sulfonyl iodide. The reaction shows high chemoselectivity for the addition of a sulfonyl group to an α-carbon over a hydroxyl group. In addition, the given protocol provides good to excellent yields of β-keto sulfones under mild reaction conditions. Moreover, the regiochemical aspect of the protocol is also explored.
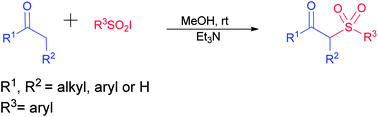

 Please wait while we load your content...
Please wait while we load your content...