 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical tweaking of a non-fluorescent GFP chromophore to a highly fluorescent coumarinic fluorophore: application towards photo-uncaging and stem cell imaging†
Tanmay
Chatterjee
,
Debjit
Roy
,
Ananya
Das
,
Anup
Ghosh
,
Partha Pratim
Bag
and
Prasun K.
Mandal
*
Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER)-Kolkata, Mohanpur Campus, West-Bengal 741252, India. E-mail: prasunchem@iiserkol.ac.in
First published on 14th October 2013
Abstract
Three step chemical tweaking of nonfluorescent parent GFP chromophore has yielded a three hundred times as bright coumarinic fluorophore. A fluorogenic compound has been prepared which after photo-uncaging yields the same highly bright fluorophore that could be used for live stem cell imaging.
The quest to enhance the fluorescence quantum yield of nonfluorescent GFP (green fluorescent protein)1,2 chromophores (p-HBDI, see Scheme 1) has engaged many researchers from biology and chemistry for more than a decade. Strong deviation of fluorescence quantum yield (ϕ) of p-HBDI (ϕ < 0.001) from that of wild type GFP (ϕ ∼ 0.8) has been noted in solution.3,4 Different mechanisms have been put forward as plausible reasons for the low fluorescence quantum yield of GFP chromophore.5,6 Several successful attempts7,8 to increase ϕ of GFP chromophore by restricting the torsional motion include mimicking GFP barrel encapsulation in human serum albumin, in octaacid cavitand, and using truncated or split GFP technique, and selective complexation with RNA aptamers, complexation with metal salts (such as Zn2+) or BF2 moiety. However, concerns have been raised regarding their usage due to lability of metal–BF2 complexes in solution.9 Different chemical modifications/substitutions in benzene and imidazole ring of p-HBDI have been tried but so far a maximum of only ∼10% fluorescence quantum yield could be achieved in GFP chromophore analogues.10 Thus, the quest for chemically modified GFP chromophore analogue with enhanced fluorescence quantum yield is still very much active.
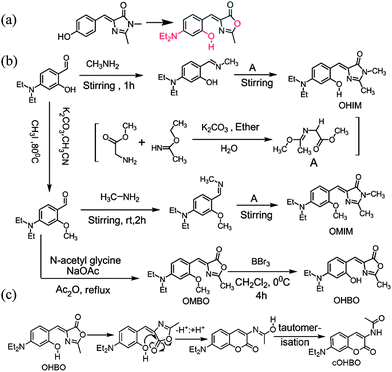 | ||
| Scheme 1 (a) Three step chemical tweaking (in red) of p-HBDI. (b) Chemical structure and synthesis of all four dyes. (c) Possible pathway for conversion of OHBO to cOHBO. | ||
Photoactivation process provides spatial and temporal control over the release of desired chemical11 and thus photoactivable compounds and fluorescent proteins are quite important towards bioimaging.12 There are quite a few number of photoactivable compounds, however, there is only one report so far where a photoactivable compound has been made with GFP chromophore analogue.13
In this manuscript we describe how three step chemical tweaking of non-fluorescent parent GFP chromophore (p-HBDI) could lead to a highly fluorescent coumarinic fluorophore (Scheme 1). Three modifications that has been incorporated are (i) p-hydroxy has been replaced by o-hydroxy, (ii) N,N-diethylamino group has been introduced at the p-position, and (iii) sp3 nitrogen in imidazole ring has been replaced by oxygen. In this direction we have made four compounds: 4-(2-hydroxy-4-N,N-diethylamino-benzylidene)-1,2-dimethyl-1H-imidazol-5(4H)-one (OHIM), 4-(2-methoxy-4-N,N-diethylamino-benzylidene)-1,2-dimethyl-1H-imidazol-5(4H)-one (OMIM) and two of their respective point atom mutation analogues (OHBO and OMBO respectively) where the nitrogen atom at 1-position have been replaced by oxygen atom (see Scheme 1b) (for synthesis and characterisation see ESI†). Absorption and emission spectra of OHIM and OHBO in different solvents are shown in ESI† and the photophysical parameters are depicted in Table 1. (For OMIM and OMBO see ESI†).
| Dye | Solvent | λ max (nm) | ε (M−1 cm−1) | ϕ | τ |
|---|---|---|---|---|---|
| a Emission maximum, absorption maximum (nm) in parentheses. | |||||
| OHIM | Toluene | 484(448) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
2.2 × 10−3 | 0.76, 32.8 ps |
| Methanol | 505(451) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
1.4 × 10−3 | 0.73, 13.9 ps | |
| cOHBO | Toluene | 444(377) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
0.90 | 3.27 ns |
| Methanol | 485(392) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.70 | 3.88 ns | |
Surprisingly, we have noticed that ϕ as well as the fluorescence lifetime (τ) of OHBO are drastically different from that of OHIM (or other two derivatives). To the best of our knowledge this is the first example of chemical modification of GFP chromophore leading to a highly bright fluorescent dye with near unity fluorescence quantum yield, i.e. more than 300 fold fluorescence enhancement from the GFP chromophore in solution. It was observed that the fluorescence lifetime of OHIM, OMIM, and OMBO are in femtosecond or picosecond time scale, whereas fluorescence lifetime of OHBO is in nanosecond time scale. Thus an enhancement of 100 to 4000 times of τ is noted for OHBO.
Such an enhancement of fluorescence quantum yield and fluorescence lifetime made us rethink what could be the possible reason and hence we had to look deeper into the spectroscopic characterisation. Based on careful analysis we propose here that although OHBO is produced (previous literature reports suggest that hydrolysis of methoxy group with BBr3 yields hydroxy group)10b in the reaction but it undergoes an intramolecular rearrangement and hence formation of a coumarinic structure is a possibility (Scheme 1c). So, OHBO plausibly remains not in hydroxyl derivative state but in coumarinic structure (cOHBO). This proposition has been supported by IR, and NMR spectroscopic results. For rearrangement, presence of –OH group at ortho position and oxygen in oxazole ring are necessary conditions. For OHIM, OMIM or OMBO these conditions are not fulfilled and thus no intramolecular rearrangement is possible. Thus, fluorescence properties of OHIM, OMIM, and OMBO are similar to each other but quite different from that of cOHBO. Absorption maximum of cOHBO matches with that of many other coumarinic probes that absorb at around 375–400 nm.14 The best proof in support of coumarinic structure stems from the single crystal X-ray structure of cOHBO (see Fig. 1). (For lattice parameters etc. see ESI†).
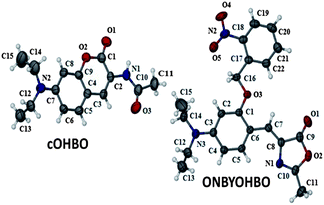 | ||
| Fig. 1 Molecular structure of cOHBO and ONBYOHBO. Thermal ellipsoids were drawn at the 50% probability level. | ||
It is quite interesting to note that at room temperature intramolecular rearrangement (Scheme 1c) is spontaneous (i.e. not externally photoinduced or thermo-induced). Thus, it is evident that three step tweaking especially the last step of point atom mutation in OHIM (N being replaced by O) followed by rearrangement and thus forming cOHBO is responsible for the much improved fluorescence properties. Most of the bright dyes available are either coumarin, rhodamine based. Hereby we have converted nonfluorescent GFP chromophore to fluorescent coumarinic dye. This methodology can be used for making many other nonfluorescent fluorescent protein chromophore to a bright fluorescent one. Work in this direction is currently underway.
Photoactivable fluorophores and fluorescent proteins11,12 are quite important towards understanding the cellular processes using different imaging techniques. Exploiting the idea of spontaneous rearrangement of OHBO leading to cOHBO (see Scheme 1c) and hence highly bright fluorescence; we have introduced an o-nitrobenzoyl group (see Scheme 2) in OHBO. Details of synthesis, and characterization of ONBYOHBO is given in ESI.† This photoactivable compound could be cleaved by light (at ∼370 nm) leading to formation of highly fluorescent cOHBO.
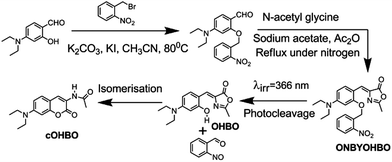 | ||
| Scheme 2 Synthesis of ONBYOHBO and its photocleavage to cOHBO. | ||
The fact that indeed cOHBO is formed because of photocleavage of ONBYOHBO (see Fig. 1) is confirmed by fluorescence spectroscopy. Steady state emission spectrum of cOHBO matches quite well with that of the photo-irradiated product (see ESI†). A better proof is shown in Fig. 2. Fluorescence decay curve and the decay parameters of cOHBO (see ESI†) and that of the photoirradiated product match quite well.
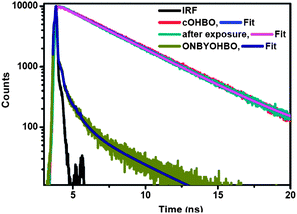 | ||
| Fig. 2 Fluorescence decay of ONBYOHBO, irradiated photoproduct, and cOHBO in DMF. λex = 377 nm. | ||
This conclusively proves that cOHBO is formed from ONBYOHBO. The decay pattern of cOHBO is clearly different from photoactivable ONBYOHBO, thus, the former could be spectroscopically distinguished easily from the latter. Earlier reports of introduction of o-nitrobenzoyl group to GFP chromophore yielded nonfluorescent product after photocleavage.13 Our approach has yielded more than 300 times brighter fluorescent product (cOHBO) than that in the reported literature.
Imaging of live cancerous cells like HeLa, U2OS with fluorescent dyes is quite common. However, non-invasive in vivo stem cell imaging15 is more modern and quite important towards regenerative medicinal therapy towards curing incurable diseases like cancer, Alzheimer disease, Parkinson disease etc. Fluorescence imaging of stem cells have so far been done using fluorescent proteins, quantum dots etc. Application of fluorescent dyes for live stem cell imaging is at its nascent stage. Thus we thought of using cOHBO for this purpose. To our delightful surprise we could image live stem cells using cOHBO (Fig. 3). (Details of imaging can be found in ESI†). We strongly believe that it is an important step towards using fluorescent dyes for stem cell imaging. In recent future many more dyes will be tried to improve the applicability of fluorescent dyes towards stem cell imaging.
 | ||
| Fig. 3 Live stem cell imaging with cOHBO. Left one is fluorescence, middle one is DIC and the right one is the merged image. | ||
To conclude, we have shown that by three point chemical tweaking parent GFP chromophore can be converted to coumarinic fluorophore with more than three hundred times enhanced fluorescence quantum yield and lifetime. This coumarinic probe could be successfully employed in live cell imaging. Employing this idea a photocleavable compound has been made which after photocleavage yields same coumarinic fluorophore. Employing our methodology many more nonfluorescent protein chromophores can be converted to highly fluorescent fluorophores. Moreover, using the photoactivation strategy highly bright fluorophores emitting at different wavelength ranging from blue to red can be prepared.
PKM thanks IISER-Kolkata for instrumental facilities and financial help. Support from the Fast-Track Project (SR/FT/CS-52/2011) of DST-India is gratefully acknowledged. TC, DR, AD, PPB thank CSIR, and AG thanks UGC for their fellowship. PKM acknowledges Professor P. Ramamurthy (NCUFP), Malancha Ta (IISER-K) for Femtosecond decay measurement, and Stem cell imaging respectively.
Notes and references
- (a) R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509–544 CrossRef CAS PubMed; (b) Green fluorescent protein: properties, application, and protocols, ed. M. Chalfie and S. R. Kein, Wiley Interscience, New Jersey, 2nd edn, 2006 Search PubMed.
- (a) M. Zimmer, Chem. Rev., 2002, 102, 759–781 CrossRef CAS PubMed; (b) R. N. Day and M. W. Davidson, Chem. Soc. Rev., 2009, 38, 2887–2921 RSC; (c) M. Zimmer, Chem. Soc. Rev., 2009, 38, 2823–2832 RSC.
- K. Y. Chen, Y. M. Cheng, C. H. Lai, C. C. Hsu, M. L. Ho, G. H. Lee and P. T. Chou, J. Am. Chem. Soc., 2007, 129, 4534–4535 CrossRef CAS PubMed.
- (a) M. Vengris, I. H. M. van Stokkum, X. He, A. F. Bell, P. J. Tonge, R. van Grondelle and D. S. Larsen, J. Phys. Chem. A, 2004, 108, 4587–4598 CrossRef CAS; (b) S. R. Meech, Chem. Rev., 2009, 38, 2922–2934 RSC.
- (a) W. Weber, V. Helms, J. A. McCammon and P. W. Langhoff, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 6177–6182 CrossRef CAS; (b) P. Altoe, F. Bernardi, M. Garavelli, G. Orlandi and F. Negri, J. Am. Chem. Soc., 2005, 127, 3952–3963 CrossRef CAS PubMed; (c) A. Usman, O. F. Mohammed, E. T. J. Nibbering, J. Dong, K. M. Solntsev and L. M. Tolbert, J. Am. Chem. Soc., 2005, 127, 11214–11215 CrossRef CAS PubMed; (d) S. S. Stavrov, K. M. Solntsev, L. M. Tolbert and D. J. Huppert, J. Am. Chem. Soc., 2006, 128, 1540–1546 CrossRef CAS PubMed; (e) J. Dong, K. M. Solntsev, O. Poizat and L. M. Tolbert, J. Am. Chem. Soc., 2007, 129, 10084–10085 CrossRef CAS PubMed; (f) J. S. Yang, G. J. Huang, Y. H. Liu and S. M. Peng, Chem. Commun., 2008, 1344–1346 RSC; (g) S. Olsen and S. C. Smith, J. Am. Chem. Soc., 2008, 130, 8677–8689 CrossRef CAS PubMed; (h) A. Baldridge, S. R. Samanta, N. Jayaraj, V. Ramamurthy and L. M. Tolbert, J. Am. Chem. Soc., 2010, 132, 1498–1499 CrossRef CAS PubMed; (i) A. Baldridge, S. R. Samanta, N. Jayaraj, V. Ramamurthy and L. M. Tolbert, J. Am. Chem. Soc., 2011, 133, 712–715 CrossRef CAS PubMed.
- (a) N. M. Webber, K. L. Litvinenko and S. R. Meech, J. Phys. Chem. B, 2001, 105, 8036–8039 CrossRef CAS; (b) K. L. Litvinenko, N. M. Weber and S. R. Meech, J. Phys. Chem. A, 2003, 107, 2616–2623 CrossRef CAS; (c) D. Mandal, T. Tahara and S. R. Meech, J. Phys. Chem. B, 2004, 108, 1102–1108 CrossRef CAS; (d) S. Rafiq, B. K. Rajbongshi, N. N. Nair, P. Sen and P. Ramanathan, J. Phys. Chem. A, 2011, 115, 13733–13742 CrossRef CAS PubMed.
- (a) L. Wu and K. Burgess, J. Am. Chem. Soc., 2008, 130, 4089–4096 CrossRef CAS PubMed; (b) K. P. Kent, L. M. Oltrogge and S. G. Boxer, J. Am. Chem. Soc., 2009, 131, 15988–15989 CrossRef CAS PubMed; (c) A. Baldridge, S. R. Samanta, N. Jayaraj, V. Ramamurthy and L. M. Tolbert, J. Am. Chem. Soc., 2010, 132, 1498–1499 CrossRef CAS PubMed; (d) A. Baldridge, K. M. Solntsev, C. Song, T. Tanioka, J. Kowalik, K. Hardcastle and L. M. Tolbert, Chem. Commun., 2010, 46, 5686–5688 RSC; (e) K. P. Kent and S. G. Boxer, J. Am. Chem. Soc., 2011, 133, 4046–4052 CrossRef CAS PubMed; (f) K. Do and S. G. Boxer, J. Am. Chem. Soc., 2011, 133, 18078–18081 CrossRef CAS PubMed; (g) A. Baldridge, S. Feng, Y. T. Chang and L. M. Tolbert, ACS Comb. Sci., 2011, 13, 214–217 CrossRef CAS PubMed; (h) J. S. Paige, K. Y. Wu and S. R. Jaffrey, Science, 2011, 333, 642–646 CrossRef CAS PubMed.
- M. S. Baranov, K. A. Lukyanov, A. O. Borissova, J. Shamir, D. Kosenkov, L. V. Slipchenko, L. M. Tolbert, I. V. Yampolsky and K. M. Solntsev, J. Am. Chem. Soc., 2012, 134, 6025–6032 CrossRef CAS PubMed.
- J. Kang, G. Zhao, J. Xu and W. Yang, Chem. Commun., 2010, 46, 2868–2870 RSC.
- (a) P. E. Ivashkin, I. V. Yampolsky and K. A. Lukyanov, Russ. J. Bioorg. Chem., 2009, 35, 726–743 CrossRef CAS; (b) W. T. Chuang, C. C. Hsieh, C. H. Lai, C. H. Lai, C. W. Shih, K. W. Chen, W. Y. Hung, Y. H. Hsu and P. T. Chou, J. Org. Chem., 2011, 76, 8189–8202 CrossRef CAS PubMed.
- (a) D. Maurel, S. Banala, T. Laroche and K. Johnsson, ACS Chem. Biol., 2010, 5, 507–516 CrossRef CAS PubMed; (b) P. Klan, T. Solomek, G. C. Bochet, A. Blanc, R. Givens, M. Rubina, V. Popik, A. Kostikov and J. Wirz, Chem. Rev., 2013, 113, 119–191 CrossRef CAS PubMed; (c) Special issue, Photochem. Photobiol. Sci., 2012, 11, 433–600 Search PubMed.
- (a) G. H. Patterson and J. Lippincott-Schwartz, Science, 2002, 297, 1873–1877 CrossRef CAS PubMed; (b) R. Ando, H. Hama, M. Yamamoto-Hino, H. Mizun and A. Miyawaki, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12651–12656 CrossRef CAS PubMed; (c) H. Tsutsui, S. Karasawa, H. Shimizu, N. Nukina and A. Miyawaki, EMBO Rep., 2005, 6, 233–238 CrossRef CAS PubMed; (d) S. Habuchi, R. Ando, P. Dedecker, W. Verheijen, H. Mizuno, A. Miyawaki and J. Hofkens, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9511–9516 CrossRef CAS PubMed; (e) N. Gagey, M. Emond, P. Neveu, C. Benbrahim, B. Goetz, I. Aujard, J. B. Baudin and L. Jullien, Org. Lett., 2008, 10, 2341–2344 CrossRef CAS PubMed; (f) E. Betzig, G. H. Patterson, R. Sougrat, O. W. Lindwasser, S. Olenych, J. S. Bonifacino, M. W. Davidson, J. Lippincott-Schwartz and H. F. Hess, Science, 2006, 313, 1642–1645 CrossRef CAS PubMed.
- D. Groff, F. Wang, S. Jockusch, N. J. Turro and P. J. Schultz, Angew. Chem., Int. Ed., 2010, 49, 7677–7679 CrossRef CAS PubMed.
- U. Brackmann, Lambdachrome Laser Dyes Lambda Physik, Goettingen, Germany, 1986 Search PubMed.
- (a) Y. Gao, J. K. Y. Chan and C. Xu, Am. J. Nucl. Med. Mol. Imaging, 2013, 3, 232–246 CAS; (b) M. R. Porcel, J. C. Wu and S. S. Gambhir, Molecular imaging of stem cells (July 30, 2009), StemBook, ed. The Stem Cell Research Community, StemBook, DOI:10.3824/stembook.1.49.1.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra44034f |
| This journal is © The Royal Society of Chemistry 2013 |
