Production of platform molecules from sweet sorghum†
Gyula
Novodárszki
a,
Nóra
Rétfalvi
a,
Gábor
Dibó
a,
Péter
Mizsey
bc,
Edit
Cséfalvay
*b and
László T.
Mika
*ab
aInstitute of Chemistry, Eötvös Loránd University, Budapest, Hungary
bDepartment of Chemical and Environmental Process Engineering, Budapest University of Technology and Economics, Budapest, Hungary. E-mail: laszlo.t.mika@mail.bme.hu; csefalvay@ch.bme.hu; Fax: +36 1 463 3197; Tel: +36 1 463 1263 Tel: +36 1 463 2035
cResearch Institute of Chemical and Environmental Process Engineering, University of Pannonia, Veszprém, Hungary
First published on 15th November 2013
Abstract
This study proves that the non-food dedicated sweet sorghum (Sorghum bicolor) can be a possible source and/or raw material of platform molecules such as levulinic acid (LA) and 5-hydroxymethyl-2-furaldehyde (5-HMF). The high sugar-containing juice derived from sweet sorghum can be efficiently converted to LA and 5-HMF by using microwave dielectric heating. Centrifugal separation was proposed as a first step of the technology to remove the insoluble materials (fibers, starch, and sand) to obtain high sugar containing feedstock for acid-catalyzed dehydration. The effects of pretreatment by centrifugal separation and reaction conditions (irradiation time, acid concentration and reaction temperature) on the formation of levulinic acid were studied. The conversions were monitored by in situ NMR spectroscopy. It was shown that maximum yield (31.4%) of LA was achieved in the presence of 2 M sulfuric acid by applying 30 min irradiation at 160 °C to the sorghum sample treated for 20 min in a centrifuge at a rotational force of 5870. It was also revealed that 5-HMF can be produced from the sweet sorghum juice in the presence of 0.05 M sulfuric acid at 120 °C.
Introduction
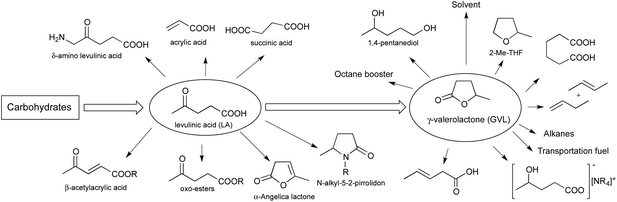
The carbon-based products of the chemical industry and more than 90% of our total energy needs are almost exclusively produced from non-renewable resources e.g. fossil fuels.1 The quality of life of mankind depends on whether we can supply the increasing population with enough goods including carbon-based consumer products. Although, it is difficult to predict the exact date of depletion of crude oil and natural gas, the development of alternative strategies to supply fuels and carbon-based feedstock should be accelerated.2 The global efforts to reduce carbon dioxide emission also demand new and innovative strategies for the green production of fuels,3 and value-added chemicals.4 In addition, nature can help to convert carbon dioxide to biomass, one of the most preferred alternative and renewable resources. Recently, the conversion of carbohydrates, the main part of biomass, has received increasing interest as a part of the supply chain to chemical products.5 Carbohydrates form the largest fraction of terrestrial biogenic renewable resources which can be converted into various platform molecules. However, only the non-edible biomass components can be considered for this purpose.6 One of the promising approaches is the conversion of the residual biomass and/or waste7,8 into platform chemicals such as 5-hydroxymethylfurfural (5-HMF) and its derivatives i.e. levulinic acid (LA) and γ-valerolactone (GVL). Recently, the valorisation of food waste,7,9 bio-oils10 as alternative feedstock was also highlighted.It was established, that saccharose and fructose can be converted to various C5-oxygenates via LA,11 which is one of the most important intermediates of the biomass-based carbon cycle.12 Due to its reactive functional groups, LA has been identified as a valuable multipurpose building block of functionalized C3–C6 oxygenates (Fig. 1).13,14
Various esters of LA are used as gasoline and biodiesel additives. δ-Aminolevulinate is a known biodegradable herbicide. The bisphenol derivative of LA (diphenolic acid) may be an interesting substitute for bisphenol-A in polymer manufacturing,13 and its calcium salt can be used as intravenous injection.15 Obviously, the most important LA derivative is GVL, which can be considered as a sustainable liquid12 and can be easily produced from LA by catalytic hydrogenation.16 GVL has been successfully used for the production of transportation fuels,17 butane isomers,17b octane booster,12 alkanes,11 pentane-1,4-diol,11,16a ionic liquids,18 adipic acid,19 polymers,20 pentanoic acid,21 or as a solvent22 (Fig. 1).
Horváth and co-workers demonstrated that saccharose was degraded to LA in 1.8 M sulfuric acid or in the presence of Nafion-NR50 with average yield of 40%.11 Since the seminal papers of Gedye23 and Giguere,24 microwave dielectric heating has become a popular technique for chemical transformations. It has been shown, that controlled microwave heating dramatically decreases reaction time.25 Our recent study demonstrated that carbohydrates of both plant and animal origin can be selectively converted to LA by using microwave irradiation with yields over 34%.26 A novel route for the microwave-assisted conversion of chitosan was published. In the presence of different Lewis acids in water, chitosan was converted to LA and 5-HMF with an average yield of 25%.27 Although, several papers were published on the acid-catalyzed degradation of carbohydrates, only a few of them focused on the utilization of crop resources. It was showed that, in the presence of diluted hydrochloric acid, LA (yield: 23%) and GVL (yield: 17%) were obtained by the transformation of giant reed (Arundo donax L.),28 however, the use of HCl is an environmental concern because it can be easily desorbed from the reaction mixture to the atmosphere. Wheat straw was also successfully converted to LA under optimized conditions in the presence of mineral acids.29 It is noteworthy, that a recent paper30 reports the conversion of different waste biomass (poplar sawdust, tobacco chops etc.) to LA in a microwave reactor with a yield of 11–32%. Recently, the use of various forms of biomass from marine origin was reported, e.g. algal biomass (Gelidium amansii) was proposed as a promising raw material for the selective production of D-glucose, D-galactose and levulinic acid in a sulfuric acid catalyzed reaction.31 The optimized process resulted in LA with an average yield of 20%.32
Due to its high sugar content (mainly D-glucose, D-fructose and saccharose), sweet sorghum (Sorghum bicolor) could be a suitable plant as a feedstock for the production of platform molecules.33 Sugars are encapsulated in the spongy tissues of the stalk, and can be easily withdrawn in a from of juice by pressing and crushing. On the other hand, sweet sorghum is a xerophyte and can grow under adverse conditions34 resulting in high biomass yield (70–100 tons per hectare with 4–9 tons of total sugar per hectare). Their adoption to continental climate has started in the 19th century,35 and its habitat in Europe could be observed after the Second World War. At that time, it was used only for the production of sugar syrup to cover the sugar needs caused by the lack of sugar beet. Recent studies showed that carbohydrates can be withdrawn from all phases of plant processing technology, and can be utilized as a starting material for the ethanol production via fermentation.36 The generation of other chemical compounds such as lactic acid,37 butanol36c acetone, various lipids, hydrogen gas, and methane from sorghum juice is also gaining more importance due to the combination of biorefinery principles with a promising feedstock.38 So far, the successful production of levulinic acid from high sugar-containing sweet sorghum (Sorghum bicolor) juice has not been published.
We report here the production of levulinic acid from sweet sorghum juice (having high sugar content but not used in food industry) by using sulfuric acid and environment-friendly microwave dielectric heating.
Results and discussion
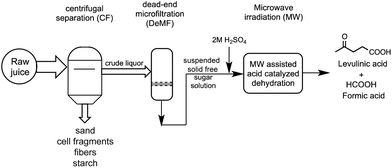
Integrated processing of sweet sorghum workup
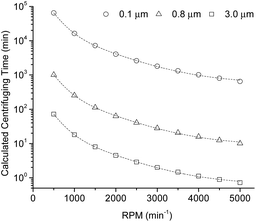
To obtain juice with the highest water-soluble carbohydrate content from raw sweet sorghum, the husked stem was crushed, filtered through a coarse sieve, and frozen on site after harvesting. This juice was kept at −18 °C until further processing to avoid self-fermentation, thus obtaining a sugar-rich juice. The remaining bagasse has high lignocellulose content and is still in focus of interest for further use.The frozen raw juice contained carbohydrate (pressed out from inside of the stem), sand particles (from the outer layer of the stem), and small plant cell fragments (due to crushing). Water-insoluble starch was also eliminated during pressing. By using five parallel lyophilized samples, the total dry solid content was 197 ± 5.7 g L−1. All floating solid materials were removed from the liquid by centrifugation. The effect of centrifugal separation time, rotational speed (i.e. relative centrifugal force and damping) on the residue content (determined in wt% of feed of centrifugal separation) was studied. Preliminary experiments showed that forced damping re-mixed the settled residue causing high turbidity of the liquor. Henceforth, unforced damping was applied in all experiments. The residue contained sand, water insoluble carbohydrates including starch, and cell fragments. Engineering calculations were applied to plan the further centrifugal separation based on the type of ingredients. Centrifugal separation time (t) is determined by the particle diameter (d) are to be eliminated according to eqn (1) assuming settling in the Stokes region.
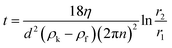 | (1) |
The centrifugal separation experiments were evaluated by using both visual evaluation and calculation of total solute content of the sample. The residue content was higher when higher rotational speed (i.e. the highest at maximum rotational centrifugal force of 5870) was applied. In general, by increasing the centrifugal separation time the ratio of the residue and the liquor increases. However, by increasing the contact time of settled residue and clarified liquor in equilibrium state the back diffusion through the boundary layer increases the turbidity of the liquor. Based on engineering considerations, 20 min was selected as the optimal centrifuging time for the optimal separation. The average residue content of centrifugal separation was 3.9 wt%, the feed weight and the total dry biomass content of the purified liquor was 171 ± 5.0 g L−1. The total carbohydrate content of the crushed sweet sorghum encapsulated in the spongy tissue of the stem accumulated in the raw juice. Centrifugation is of assistance to eliminate the dissolved content of spongy tissues by lowering its density and resulting in floating cell fragments in the clarified liquor. These floating fragments can be easily eliminated later by dead-end filtration through a 15 μm pore-size filter paper. The influence of the centrifuging time on the total content of the dry biomass and the turbidity of sweet sorghum juice is shown in Table 1. Due to the 20 min centrifugation, the turbidity was significantly decreased (entries 1 and 2, 94.5%) by removing the suspended solids which means a loss of 13.2% TDS of the liquor. By doubling the centrifuging time (entry 3, 40 min), the turbidity was further decreased by one order of magnitude. In spite of it, TDS slightly increased. Presumably, the application of longer treatment time results in the destruction of cells, consequently intensifies the release of carbohydrates from the cell body to the liquor. The residue was found to be constant (2.4 ± 0.2 wt%) indicating that 20 min was sufficient to remove all the suspended solids. It should be noted, that the total dissolved solid i.e. sugar content, according to the water content of plant, depends on the annual growing conditions such as rainfall, sunshine hours in growth-time and composition of the soil, etc.
To conclude, the purified liquor obtained by 20 min centrifugal purification is a valuable feedstock for production of levulinic acid. The effect of the centrifugal separation time and the reaction parameters (temperature, reaction time) on the formation and yield of levulinic acid was systematically studied.
Hereby, a possible route for the production of levulinic acid and formic acid from sweet sorghum is proposed (Fig 3).
Microwave-assisted conversion of carbohydrates
The catalytic dehydration of non-edible carbohydrates to levulinic and formic acids via 5-HMF is a particularly attractive approach for carbohydrate-based biomass conversion.6,26–32,41 Formic acid can be efficiently decomposed to CO2 and H2 by using phosphine modified iron catalyst,42 the hydrogen gas evolved can be used for the reduction of LA to GVL. Alternatively, transfer hydrogenation of LA with HCOOH, as a hydrogen source, in the presence of Shvo's catalyst results in the formation of γ-hydroxyvaleric acid, which spontaneously turns to GVL via ring closure dehydration.43 Consequently, the production of LA is an essential step in the sustainable conversion of biomass.The effect of different reaction conditions (temperature, irradiation time) on the efficiency of the dehydration was systematically studied. The optimization of the reaction conditions on the formation of LA from model substrates (D-glucose, saccharose, D-fructose, cellulose etc.) was recently reported by Szabolcs et al.26 It was shown that the highest yield was achieved by using 2 M sulfuric acid as catalyst. Comparing the conventional and dielectric heating, the reaction time was significantly shorter and yields were always higher when MW heating was applied. The effect of the temperature on the LA formation in the presence of 2 M H2SO4 was determined in the range of 100–200 °C at 15, 30 and 45 min irradiation times. Initially, the crude sweet sorghum juice containing fibers, starch, cell walls etc. with a TDS content of 197 g L−1 was used. Expectedly, working at 100 and 120 °C low conversions were observed. At higher temperature the formation of LA slowly increased reaching a maximum (9.7 wt%) at 160 °C for 15 min (Fig. 4). Over 160 °C, the humin or tar formation became more significant indicated by lower yields and darker color of the reaction mixture.
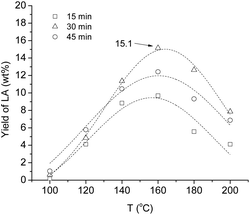 | ||
| Fig. 4 Influence of the reaction temperature and irradiation time on the yield of interconversion of crude sweet sorghum juice to levulinic acid in 2 M H2SO4 (ESI Table S1†). | ||
When the reaction time was doubled (30 min), the yield increased to 15.1 wt% under identical conditions. However, when the irradiation time was tripled, the product formation decreased (45 min, 12.4% at 160 °C). Although, the optimal conditions (160 °C, 30 min) were similar to those of obtained for pure model compounds, the yields were significantly lower (cf. Szabolcs et al.ref. 26). Presumably, large amount of interconvertible carbohydrates were allocated in the spongy tissues of stem, which were released by crushing the cell wall. Accordingly, the effect of centrifuging time on the LA formation was studied. When the raw juice was centrifuged for 20 min, the turbidity of the sample decreased significantly. However, a decrease in TDS was observed, as well (Table 1, entries 1 and 2). This sample contained 171 g L−1 of TDS, when it was treated with 2 M sulfuric acid, significantly higher amount (31.4 wt%) of LA was formed (Fig. 5a).
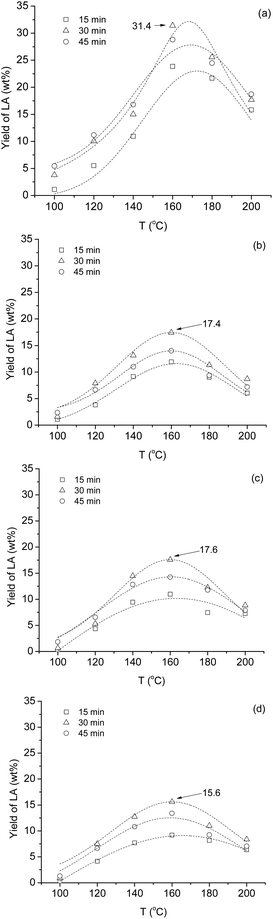 | ||
| Fig. 5 Influence of the reaction temperature and irradiation time on the yield of interconversion of crude sweet sorghum juice to levulinic acid in 2 M H2SO4. (a) tcentrif. = 20 min (171 g L−1; ESI Table S2†); (b) tcentrif. = 40 min (186 g L−1; ESI Table S3†); (c) tcentrif. = 60 min (196 g L−1, ESI Table S4†); (d) tcentrif. = 90 min (202 g L−1, ESI Table S5†). | ||
Expectedly, similar optimal conditions were observed. If 1.5 M sulfuric acid was used, significantly lower amount (18.4 wt%) of LA was formed. By using juice centrifuged for 40 min having TDS of 186 g L−1, the maximum yield dropped to 17.4 wt% under identical conditions (Fig. 5b). Further increase in centrifuging time had no influence on the final concentration of LA: maximum yields were 17.6 wt% for 60 min and 15.6 wt% for 90 min treated samples (Fig. 5c and d) under the optimal conditions. To conclude, 20 min centrifuging time was enough to purify the raw juice, at the same time the highest yield of LA was obtained. Importantly, the best yield was a slightly higher than that of obtained for the conversion of pure model monosaccharides (27 ± 0.5%).26 The TDS contains different forms of polysaccharide-based cell fragments in colloidal state, or other organic and inorganic compounds such as KCl. The ash content of the juice was in the range of 0.35–0.6%, as expected.44 Kerton and co-workers investigated the effect of addition of different chlorine containing salts on the degradation of N-Ac-D-glucosamine. It was established, that the presence of chlorine containing salts accelerates the hydrolysis and/or reduces the side reactions, which can result in slightly higher product yield.45 Similar observation was made in the case of chlorine-based ionic liquids,46 and during hydrolysis of chitosan under microwave irradiation.47 Consequently, naturally present KCl can increase the yield.
Conventional heating and microwave irradiation for the production of levulinic acid were also compared. To obtain similar yield (28 wt%), under conventional heating at 160 °C, the reaction time should be doubled, in accordance with literature data.25
Since the influence of different insoluble and/or colloidal state components remained in the solution after centrifugation on the acid catalyzed dehydration is unknown, dead-end microfiltration (DeMF) with 15 μm pore-sized filter paper was applied to remove the floating fragments (microscopic size fibers, cell fragments etc.) before sulfuric acid was added. Two 30 min centrifuged samples were treated under optimal conditions resulting in a yield of 32.1 wt% for non-filtered and of 31.5 wt% for DeMF. It clearly indicates, that DeMF has negligible influence on the LA formation. Interestingly the LA yield obtained for the 30 min centrifuged sample was similar to that of the 20 min one.
In order to investigate the effect of scale-up on LA formation, 50 mL sorghum juice (centrifuged for 20 min) was treated with 2 M sulfuric acid under optimal conditions (160 °C, 30 min) resulting in LA with a yield of 29.5 wt%. Expectedly, no significant decrease in LA production was observed when microwave was applied.
In situ spectroscopic studies
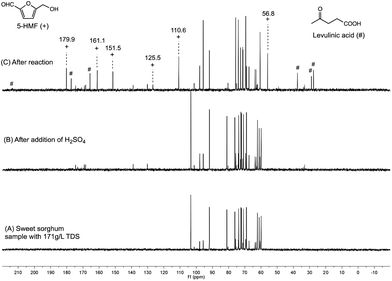
In situ spectroscopy could provide the molecular level information under reaction conditions in real time to structurally identify key species and develop the reaction network between substrates, intermediates, products, and side-products.48The form of dissolved sugars in the sweet sorghum juice (171 g L−1 TDS) was identified by NMR spectroscopy. The 13C{1H}-NMR spectra indicated the presence of two monosaccharides (D-fructose and D-glucose) and one disaccharide (saccharose) with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (Fig. 6, spectrum A), no other carbohydrate species were detected. The same ratio was obtained by using enzymatic assay. The rapid cleavage of the glycosidic bond of saccharose to D-fructose and D-glucose took place after the addition of sulfuric acid at room temperature. It was indicated by the disappearance of the peaks of saccharose (▽) at 103.9, 81.1, 76.2, 61.2, and 59.9 ppm, at the same time with the increasing intensity of the peaks of D-fructose (*) and D-glucose (○) (Fig. 6, spectrum B). By applying microwave irradiation at 160 °C for 30 min, the sugar content was transformed to levulinic acid (#) and formic acid (Fig. 6, spectrum C). Although, the 13C-NMR spectrum did not indicate any water soluble by-products, moderate tar formation could be detected. The brownish residue (which can be dissolved in EtOAc) consists of high carbon containing humins. It was proposed, that these oligomers and polymers might be formed by the H+ catalyzed oligomerization of the C
5 (Fig. 6, spectrum A), no other carbohydrate species were detected. The same ratio was obtained by using enzymatic assay. The rapid cleavage of the glycosidic bond of saccharose to D-fructose and D-glucose took place after the addition of sulfuric acid at room temperature. It was indicated by the disappearance of the peaks of saccharose (▽) at 103.9, 81.1, 76.2, 61.2, and 59.9 ppm, at the same time with the increasing intensity of the peaks of D-fructose (*) and D-glucose (○) (Fig. 6, spectrum B). By applying microwave irradiation at 160 °C for 30 min, the sugar content was transformed to levulinic acid (#) and formic acid (Fig. 6, spectrum C). Although, the 13C-NMR spectrum did not indicate any water soluble by-products, moderate tar formation could be detected. The brownish residue (which can be dissolved in EtOAc) consists of high carbon containing humins. It was proposed, that these oligomers and polymers might be formed by the H+ catalyzed oligomerization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double-bond-containing intermediates.49 Presumably, the peak at 130–140 ppm (Fig. 6B) could belong to these species. The calculated yield of LA was 31.1 wt%, verifying the reproducibility of the dehydration. Noteworthy, the NMR spectrum did not indicate the presence of 5-HMF. It can be explained by the fast decomposition of 5-HMF under acidic conditions (0.1–2 M), the conversion of 5-HMF to LA is much faster than its formation from fructose. Considering the mass balance of 89.5 wt%, the starting materials could be encountered as products and by-products (ESI Fig. S3†). Importantly, the filtered and dried brownish-black tar (121.7 mg) was insoluble in common solvents. The elementary analysis of tar showed more than 98% carbon content. The pyrolysis resulted in 4% solid residue, no melting could be observed. In order to re-establish the transformation of carbohydrate mixture to LA, an authentic sample of 4 mL solution of D-fructose (29.8 mg, 0.165 mmol), D-glucose (29.8 mg, 0.165 mmol), and saccharose (283 mg, 0.827 mmol) was hydrolyzed in the absence of sweet sorghum juice matrix under optimised conditions and monitored by 13C{1H}-NMR. Similarly to that of the sorghum sample, the spectra indicated the acid-catalyzed decomposition of saccharose to D-fructose and D-glucose and the presence of LA with a yield of 32.6 wt% after 30 min irradiation time (ESI Fig. S2†).
C double-bond-containing intermediates.49 Presumably, the peak at 130–140 ppm (Fig. 6B) could belong to these species. The calculated yield of LA was 31.1 wt%, verifying the reproducibility of the dehydration. Noteworthy, the NMR spectrum did not indicate the presence of 5-HMF. It can be explained by the fast decomposition of 5-HMF under acidic conditions (0.1–2 M), the conversion of 5-HMF to LA is much faster than its formation from fructose. Considering the mass balance of 89.5 wt%, the starting materials could be encountered as products and by-products (ESI Fig. S3†). Importantly, the filtered and dried brownish-black tar (121.7 mg) was insoluble in common solvents. The elementary analysis of tar showed more than 98% carbon content. The pyrolysis resulted in 4% solid residue, no melting could be observed. In order to re-establish the transformation of carbohydrate mixture to LA, an authentic sample of 4 mL solution of D-fructose (29.8 mg, 0.165 mmol), D-glucose (29.8 mg, 0.165 mmol), and saccharose (283 mg, 0.827 mmol) was hydrolyzed in the absence of sweet sorghum juice matrix under optimised conditions and monitored by 13C{1H}-NMR. Similarly to that of the sorghum sample, the spectra indicated the acid-catalyzed decomposition of saccharose to D-fructose and D-glucose and the presence of LA with a yield of 32.6 wt% after 30 min irradiation time (ESI Fig. S2†).
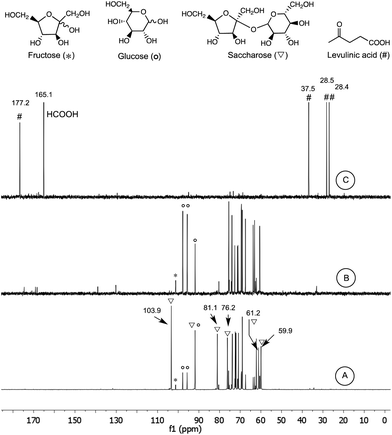 | ||
| Fig. 6 13C-NMR spectra of the interconversion of sweet sorghum juice (20 min centrifuging time) to levulinic acid and formic acid (the detailed assignments are presented in ESI Fig. S1†). (A): sample before addition of H2SO4; (B) sample after addition of H2SO4; (C): sample after the reaction. The carboxyl carbon of LA appeared at 213.5 ppm (not indicated on the presented region of spectra). | ||
Horváth drew the molecular map of the acid-catalyzed degradation of D-fructose to 5-HMF, which can further be dehydrated to levulinic acid.50 It was established, that low acid concentrations (10−5 to 10−1 M) favor the formation of 5-HMF in DMSO at 120 °C, one of the applicable solvents for the preparation of 5-HMF from carbohydrates. We have therefore investigated the possibility of the production of 5-HMF from sweet sorghum juice in aqueous media. When 50 μL of 2 M H2SO4 was added to 400 μL of pretreated sorghum juice (20 min centrifuged) followed by heating to 120 °C for 2 h, the peaks at 179.9, 161.1, 151.5, 125.5 and 110.6 ppm indicated the formation of 5-HMF (Fig. 7). Importantly, the presence of levulinic acid could also be detected (213.2, 177.1, 165.7, 37.4, 28.6, 27.6 ppm). Although, the HMF was successfully produced from sweet sorghum juice, the yields and selectivities were modest. The preliminary investigations were performed in the presence of 0.1 M and 0.05 M sulfuric acid,50 respectively. The yield of pure HMF was modest (<2%) for 30 min irradiation time at 120 °C. When irradiation time was increased, the conversion was increased; however the formation of LA was observed. It is important to note, that the formation of HMF strongly depends on the nature of the solvents. The detailed investigation of this transformation is under further studies.
The initial step of the conversion of sweet sorghum juice to levulinic acid is the fast dehydration of saccharose to equimolar amounts of D-glucose and D-fructose, which are present in equilibrium in furanose or pyranose forms. The protonation and dehydration of the fructofuranose lead to the formation of fructosyl oxocarbenium ion, which is the starting molecule of levulinic acid, through 5-HMF, or humin by-products.
Experimental
All chemicals were purchased from Sigma-Aldrich Kft., Budapest, Hungary and used without further purification. Deuterated solvents were purchased from Euriso-Top SAS, Saint-Aubin Cedex, France. Sweet sorghum was obtained from Coopinter Kft. (Budapest, Hungary).Centrifugal separation was applied by using a Rotina 380 type centrifuge (with a maximum capacity of 4 × 290 mL). The centrifugation time was set to 5, 20, 30, 40, 60, and 90 minutes at a relative centrifugal force (RCF) of 59 (minimum) or 5870 (maximum value). Damping force was set either to 0 (no forced damping) or 5 (middle-forced damping). In all cases 1 liter of the raw juice was divided into four equal portions = 4 × 250 mL by weight with maximum deviation of 0.1 g taking into account the maximum capacity of the centrifuge. After the centrifugal separation the clarified liquor was decanted and the ratio of liquor and residue was determined by weight. The remaining suspended solid content of liquor was removed by dead-end microfiltration using a 603 N type filter paper with a pore size of 15 μm.
The dynamic viscosity of the juice (η), measured by using a Hoeppler viscosimeter, was 1.76 mPa s at 20 °C. The average juice density (ρf) was 1.083 g mL−1 at 20 °C. The fix values of r2 = 0.19 m and r1 = 0.09 m were determined by the dimensions of rotor of the equipment. The ash content of the juice was determined first by heating to 105 °C to remove water, followed by pyrolysis at 800 °C.
Hydrolysis and dehydration reactions were performed in a 10 mL quartz reactor with a Milestone Multisynth AFC-FO 300 microwave instrument (Labsystem Kft., Budapest, Hungary) equipped with an automated safety release system. The Multisynth microwave reactor was always used in the single-mode option. The reactions were conducted between 100 and 200 °C by using the automatic temperature-control system of the instrument. The upper limit of the energy applied was set to 135 W and the reaction time was varied from 15 to 60 min. In a typical experiment, the solution of the sweet sorghum (1 mL) was mixed with sulfuric acid (3 mL). After completing the reaction, the reactor was cooled down to room temperature by applying air-cooling. All reactions were repeated twice in order to collect enough material for further analysis. The reaction mixture (ca. 8 mL) was filtered, the occasionally formed insoluble residue was washed with distilled water (2 × 3 mL), then with ethyl acetate (2 × 3 mL), and all filtrates were combined. The organic phase was separated, the aqueous phase was further extracted with ethyl acetate (4 × 10 mL). The combined organic phase was dried over MgSO4. After solvent removal a brownish-yellowish, viscous liquid remained.
NMR spectra were recorded in a 5 mm NMR tube using a Bruker Avance-250 spectrometer. The product analysis was performed by both 1H-NMR and 13C-NMR.
Conclusions
In conclusion, the high sugar-containing juice derived from sweet sorghum (Sorghum bicolor) can be efficiently converted to levulinic acid by using microwave dielectric heating. Centrifugal separation was proposed as a first step of the technology to remove the insoluble materials (fibers, starch, and sand) to obtain high sugar containing feedstock for acid-catalyzed dehydration. We investigated the effects of pretreatment by centrifugal separation and reaction conditions (irradiation time and reaction temperature) on the formation of levulinic acid. It was found that maximum yield of 31.4% was achieved in the presence of 2 M sulfuric acid by applying 30 min irradiation at 160 °C to the sorghum sample treated for 20 min in a centrifuge at a rotational force of 5870. The total dissolved solid content represents not only the dissolved carbohydrate content of the juice, but also the polysaccharide-based cell fragments in colloidal state, proteins, and lipids etc. The conversions were identified by in situ NMR spectroscopy. It was also revealed that 5-HMF can be produced from the sweet sorghum juice in the presence of 0.05 M sulfuric acid at 120 °C for 2 h. This study proves that the non-food dedicated sweet sorghum can be a possible source and/or raw material of platform molecules such as levulinic acid, i.e. it clearly corresponds to the concept of a biorefinery.Acknowledgements
This work was supported by the Hungarian Scientific Research Fund (Grant no. OTKA-CNK 78065). The authors thank to Coopinter Kft. for providing raw sweet sorghum. This research work was partly supported by the projects titled “Development of integrated agriculture production storage processing and logistic system for sweet sorghum” (TECH_08_A3/2-2008-0401). Portions of this work were funded Budapest University of Technology and Economics project number KMR_12-1-2012-0066.Notes and references
- K. S. Deffeyes, Beyond Oil: The View from Hubbert's Peak, Farrar, Straus and Giroux, New York, 2005 Search PubMed.
- (a) D. L. Klass, Biomass for Renewable Energy, Fuels, and Chemicals, Elsevier, Amsterdam, 1998 Search PubMed; (b) D. J. C. MacKay, Sustainable Energy – Without the Hot Air, UIT Cambridge Ltd, Cambridge, 2009 Search PubMed; (c) J. Chow, Science, 2003, 302, 1528 CrossRef PubMed.
- A. J. Ragauskas, Science, 2006, 311, 484 CrossRef CAS PubMed.
- (a) S. Fernando, S. Adhikari, C. Chandrapal and N. Murali, Energy Fuels, 2006, 20, 1927 Search PubMed; (b) G. W. Huber and A. Corma, Angew. Chem., Int. Ed., 2007, 46, 7184 CrossRef CAS PubMed; (c) J. N. Cheda, G. W. Huber and J. A. Dumesic, Angew. Chem., Int. Ed., 2007, 46, 7164 CrossRef PubMed; (d) M. Stöcker, Angew. Chem., Int. Ed., 2008, 47, 9200 CrossRef PubMed.
- (a) G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed; (b) A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411 CrossRef CAS PubMed; (c) R. A. Sheldon, Catal. Today, 2011, 167, 3 CrossRef CAS.
- I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2169 CrossRef PubMed.
- C. O. Tuck, E. Perez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695 CrossRef CAS PubMed.
- J. H. Clark, L. A. Pfaltzgraff, V. L. Budarin, A. J. Hunt, M. Gronnow, A. S. Matharu, D. J. Macquarrie and J. R. Sherwood, Pure Appl. Chem., 2013, 85, 1625 CrossRef CAS.
- (a) L. A. Pfaltzgraff, M. De bruyn, E. C. Cooper, V. Budarin and J. H. Clark, Green Chem., 2013, 15, 307 RSC; (b) C. S. K. Lin, L. A. Pfaltzgraff, L. Herrero-Davila, E. B. Mubofu, S. Abderrahim, J. H. Clark, A. A. Koutinas, N. Kopsahelis, K. Stamatelatou, F. Dickson, S. Thankappan, Z. Mohamed, R. Brocklesbyc and R. Luque, Energy Environ. Sci., 2013, 6, 426 RSC.
- (a) K. Jacobson, K. C. Maheria and A. K. Dalai, Renewable Sustainable Energy Rev., 2013, 23, 91 CrossRef CAS; (b) S. Xiu and A. Shahbazi, Renewable Sustainable Energy Rev., 2012, 16, 4406 CrossRef CAS.
- H. Mehdi, V. Fábos, R. Tuba, A. Bodor, L. T. Mika and I. T. Horváth, Top. Catal., 2008, 48, 49 CrossRef CAS.
- I. T. Horváth, H. Mehdi, V. Fábos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238 RSC.
- J. J. Bozell, L. Moens, D. C. Elliot, Y. Wang, G. G. Neuenscwander, S. W. Fitzpatrik, R. J. Biliski and J. L. Jarnefeld, Resour., Conserv. Recycl., 2000, 28, 227 CrossRef.
- B. Girisuta, L. P. B. M. Janssen and H. J. Heeres, Chem. Eng. Res. Des., 2006, 84, 339 CrossRef CAS.
- R. H. Leonard, Ind. Eng. Chem., 1956, 48, 1331 CrossRef CAS.
- (a) F. M. A. Geilen, B. Engendahl, A. Harwardt, W. Marquardt, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2010, 49, 5510 CrossRef CAS PubMed; (b) J. M. Tukacs, D. Király, A. Strádi, G. Novodárszki, Z. Eke, G. Dibó, T. Kégl and L. T. Mika, Green Chem., 2012, 14, 2057 RSC; (c) W. R. H. Wright and R. Palkovits, ChemSusChem, 2012, 5, 1657 CrossRef CAS PubMed; (d) J. M. Tukacs, R. V. Jones, F. Darvas, G. Dibó, G. Lezsák and L. T. Mika, RSC Adv., 2013, 3, 16283 RSC.
- (a) J. Q. Bond, D. Martin Alonso, R. M. West and J. A. Dumesic, Langmuir, 2010, 26, 16291 CrossRef CAS PubMed; (b) J. Q. Bond, J. A. Dumesic, D. M. Alonso, D. Wang and R. M. West, Science, 2010, 327, 1110 CrossRef CAS PubMed.
- (a) D. Fegyverneki, L. Orha, G. Láng and I. T. Horváth, Tetrahedron, 2010, 66, 1078 CrossRef CAS; (b) A. Strádi, M. Molnár, M. Óvári, G. Dibó, F. U. Richter and L. T. Mika, Green Chem., 2013, 15, 1857 RSC.
- P. K. Wong, C. Li and L. Stubbs, Patent Appl. WO 2012/134397A1, 2012.
- M. Chalid, H. J. Heeres and A. A. Broekhuis, J. Appl. Polym. Sci., 2011, 123, 3556 CrossRef.
- J.-P. Lange, R. Price, P. M. Ayoub, J. Louis, L. Petrus, L. Clarke and H. Gosselink, Angew. Chem., Int. Ed., 2010, 49, 4479 CrossRef CAS PubMed.
- I. T. Horváth, Green Chem., 2008, 10, 1024 RSC.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279 CrossRef CAS.
- R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Tetrahedron Lett., 1986, 27, 4945 CrossRef CAS.
- (a) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed; (b) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed.
- Á. Szabolcs, M. Molnár, G. Dibó and L. T. Mika, Green Chem., 2013, 15, 439 RSC.
- K. W. Omari, J. E. Besaw and F. M. Kerton, Green Chem., 2012, 14, 1480 RSC.
- A. M. R. Galletti, C. Antonetti, E. Ribechini, M. P. Colombini, N. N. O. Di Nasso and E. Bonari, Appl. Energy, 2013, 102, 157 CrossRef CAS.
- C. Chang, P. Cen and X. Ma, Bioresour. Technol., 2007, 98, 1448 CrossRef CAS PubMed.
- A. M. R. Galletti, C. Antonetti, V. De Luise, D. Licursi and N. Nassi, BioResources, 2012, 7, 1824 Search PubMed.
- G.-T. Jeong and D.-H. Park, Appl. Biochem. Biotechnol., 2009, 161, 41 CrossRef PubMed.
- M. Kang, S. W. Kim, J.-W. Kim, T. H. Kim and J. S. Kim, Renewable Energy, 2013, 54, 173 CrossRef CAS.
- DOE critical review series, ed. R. A. Nathan, Dept. of Energy, Technical Information Service, Oak Ridge, Tenn., USA, 1978 Search PubMed.
- L. Barbanti, S. Grandi, A. Vecchi and G. Venturi, Eur. J. Agron., 2006, 25, 30 CrossRef.
- C. O. Townsend, J. Hered., 1909, 5, 269 CrossRef.
- (a) M. Kim, K.-J. Han, Y. Jeong and D. F. Day, Food Sci. Biotechnol., 2012, 21, 1075 CrossRef CAS; (b) J. Yu, T. Zhang, J. Zhong, X. Zhang and T. Tan, Biotechnol. Adv., 2012, 30, 811 CrossRef CAS PubMed; (c) L. Wang, Z. Luo and A. Shahbazi, Ind. Crops Prod., 2013, 42, 280 CrossRef CAS; (d) T. Hattori and S. Morita, Plant Prod. Sci., 2010, 13, 221 CrossRef; (e) S. I. Mussatto, G. Dragone, P. M. R. Guimarães, J. P. A. Silva, L. M. Carneiro, I. C. Roberto, A. Vicente, L. Domingues and J. A. Teixeira, Biotechnol. Adv., 2010, 28, 817 CrossRef CAS PubMed.
- K. Hetényi, K. Gál, Á. Németh and B. Sevella, J. Chem. Technol. Biotechnol., 2010, 85, 872 CrossRef.
- M. B. Whitfield, M. S. Chinn and M. W. Veal, Ind. Crops Prod., 2012, 37, 362 CrossRef CAS.
- (a) http://www.engineeringtoolbox.com/particle-sizes-d_934.html ; (b) http://www.engineeringtoolbox.com/density-materials-d_1652.html .
- H. N. Dengate, D. W. Baruch, J. McKenzie, L. D. Simmons, P. Meredith and W. R. Morrison, Starch/Staerke, 1979, 31, 40 CrossRef CAS.
- (a) L. J. Diang, D.-M. Lai, Y. Fu and Q.-X. Guo, Angew. Chem., Int. Ed., 2009, 48, 6529 CrossRef PubMed; (b) P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538 RSC.
- A. Boddien, D. Mellmann, F. Gärtner, R. Jackstell, H. Junge, P. J. Dyson, G. Laurenczy, R. Ludwig and M. Beller, Science, 2011, 333, 1733 CrossRef CAS PubMed.
- V. Fábos, I. T. Horváth, N. Kaposy and H. Mehdi, Patent Appl. HU2009-00276-A1, 2009.
- B. J. Lime, Raw sugar production from sugarcane and sweet sorghum, in Tropical foods: Chemistry and Nutrition, ed. G. E. Inglett and G. Charalambous, Acad. Press, London, 1979, vol. 1, pp. 171–182 Search PubMed.
- (a) K. W. Omari, L. Dodot and F. M. Kerton, ChemSusChem, 2012, 5, 1767 CrossRef CAS PubMed; (b) M. W. Drover, K. W. Omari, J. N. Murphy and F. M. Kerton, RSC Adv., 2012, 2, 4642 RSC.
- T. Ståhlberg, S. Rodriguez-Rodriguez, P. Fristrup and A. Riisager, Chem.–Eur. J., 2011, 17, 1456 CrossRef PubMed.
- R. Xing, S. Liu, H. Yu, Z. Guo, P. Wang, C. Li, Z. Li and P. Li, Carbohydr. Res., 2005, 340, 2150 CrossRef CAS PubMed.
- (a) L. T. Mika, R. Tuba, I. Tóth, S. Pitter and I. T. Horváth, Organometallics, 2011, 30, 4751 CrossRef CAS; (b) S. Csihony, L. T. Mika, G. Vlád, K. Barta, C. P. Mehnert and I. T. Horváth, Collect. Czech. Chem. Commun., 2007, 72, 1094 CrossRef CAS; (c) Z. Pusztai, G. Vlád, A. Bodor, I. T. Horváth, H. J. Laas, R. Halpaap and F. U. Richter, Angew. Chem., Int. Ed., 2006, 45, 107 CrossRef CAS PubMed; (d) R. Tuba, L. T. Mika, A. Bodor, Z. Pusztai, I. Tóth and I. T. Horváth, Organometallics, 2003, 22, 1582 CrossRef CAS; (e) L. Qi and I. T. Horváth, ACS Catal., 2012, 2, 2247 CrossRef CAS.
- J. Horvat, B. Klaić, B. Metelko and V. Šunjić, Tetrahedron Lett., 1985, 26, 2111 CrossRef CAS.
- G. R. Akien, L. Qi and I. T. Horváth, Chem. Commun., 2012, 48, 5850 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra42895h |
| This journal is © The Royal Society of Chemistry 2014 |