 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of azetidines and pyrrolidines via iodocyclisation of homoallyl amines and exploration of activity in a zebrafish embryo assay†
Antonio
Feula
ab,
Sundeep S.
Dhillon
c,
Rama
Byravan
b,
Mandeep
Sangha
b,
Ronald
Ebanks
b,
Mariwan A.
Hama Salih
b,
Neil
Spencer
d,
Louise
Male
e,
Istvan
Magyary
f,
Wei-Ping
Deng
a,
Ferenc
Müller
c and
John S.
Fossey
*b
aSchool of Pharmacy, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China
bSchool of Chemistry, University of Birmingham, Birmingham, West Midlands B15 2TT, UK. E-mail: j.s.fossey@bham.ac.uk
cSchool of Clinical and Experimental Medicine, College of Medical and Dental Sciences, University of Birmingham, Birmingham, West Midlands B15 2TT, UK
dSchool of Chemistry, NMR Spectroscopy Facility, University of Birmingham, Birmingham, West Midlands B15 2TT, UK
eSchool of Chemistry, X-Ray Crystallography Facility, University of Birmingham, Birmingham, West Midlands B15 2TT, UK
fUniversity of Kaposvar, Faculty of Animal Science, Department of Nature Protection, H7400 Kaposvar Guba S. u. 40, Hungary
First published on 3rd July 2013
Abstract
Room temperature iodocyclisation of homoallylamines stereoselectively delivers functionalised 2-(iodomethyl)azetidine derivatives in high yield. Increasing reaction temperature from 20 °C to 50 °C switches the reaction outcome to realise the stereoselective formation of functionalised 3-iodopyrrolidine derivatives. It was shown that these pyrrolidines are formed via thermal isomerisation of the aforementioned azetidines. Primary and secondary amines could be reacted with iodomethyl azetidine derivatives to deliver stable methylamino azetidine derivatives. With subtle changes to the reaction sequences homoallyl amines could be stereoselectively converted to either cis- or trans-substituted 3-amino pyrrolidine derivatives at will. The stereochemical divergent synthesis of cis and trans substituted pyrrolidines supports an ion part, aziridinium, isomerisation pathway for azetidine to pyrrolidine isomerisation. Six azetidine derivatives were probed in a zebrafish embryo developmental assay to detect potential biological effects through the analysis of morphology and motility behaviour phenotypes. The range of effects across the probed molecules demonstrates the suitability of this assay for screening azetidine derivatives. One of the probed molecules, rac-(((cis)-1-benzyl-4-phenylazetidin-2-yl)methyl)piperidine, exhibited particularly interesting effects in the developmental assay presenting with hypopigmentation and reduced circulation amongst others. This shows that the zebrafish embryo provides a fast, sensitive and effective way to screen new compounds and in the future in combination with existing in vivo and in vitro assays it will become an integral part in drug discovery and development.
Introduction
Four and five membered saturated nitrogen containing heterocycles, azetidines and pyrrolidines respectively, are important agrochemical and pharmacological motifs. Whilst pyrrolidines are a mainstay of the pharmaceutical and natural products arenas far fewer molecules containing azetidines have been reported, despite their intriguing biological activities.1 Naturally occurring azetidine containing compounds include the siderophore mugenic acid2,3 and a marine sponge extract penaresidin A.4–6 Azetidines are often compared to pyrrolidines in terms of various biological assays, for example nicotine binds less effectively than its azetidine analogue, Fig. 1, to acetylcholine receptors.7 This coupled with studies into the psychotropic potency,8 potential CNS-focused leads in drug-discovery9 and antibacterial activity10 demonstrates that azetidine derivatives are attractive biologically active motifs.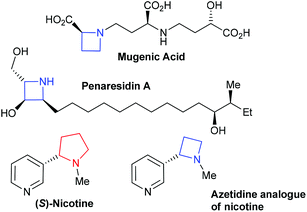 | ||
| Fig. 1 Examples of azetidine containing molecules alongside pyrrolidine containing nicotine. | ||
Results and discussion
Synthesis
In an earlier report we detailed an iodine mediated cyclisation and isomerisation protocol that delivered pyrrolidines via unstable azetidine intermediates.11 Herein new observations and understanding are presented, such as streamlined access to pyrrolidines, and exploiting the observation that simply carefully controlling reaction temperature allows direct access to either azetidines 2 or pyrrolidines 3. For example reaction of N-benzyl-1-phenylbut-3-en-1-amine (1a) with iodine and sodium hydrogen carbonate in acetonitrile at 20 °C, delivered cis-azetidine 2a in 96% yield (which is prone to isomerisation to cis-pyrrolidines), whereas running this cyclisation reaction at a slightly elevated temperature (50 °C) cleanly delivers cis-pyrrolidine 3a as the only detectable product (82% isolated yield), Scheme 1. It should be noted that the temperature should be closely controlled through manipulations since azetidines 2 were found to readily isomerise under solvent removal conditions above 20 °C.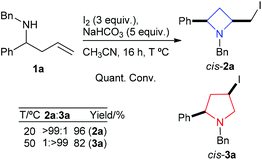 | ||
| Scheme 1 Temperature dependent product distribution in the iodine mediated cyclisation of homoallyl amine 1a. | ||
Azetidines
Having established the general synthetic protocol for the synthesis of 2,4-cis-azetidines from homoallyl amines, i.e. treatment with iodine and sodium hydrogen carbonate in acetonitrile at room temperature,11 the effect of varying the base was probed. Since iodine containing azetidines 2 are prone to isomerisation with time the post-workup mixture obtained from the reaction was used to determine conversion (proton NMR spectroscopy), post work-up isolates had already been shown by us to be excellent starting points for subsequent derivatisation.11Table 1 (entries 1 to 12) details the effect of varying the base under the standard conditions of a 16 h room temperature reaction of homoallyl amine 1a with three equivalents of iodine in acetonitrile. Whilst initially selected sodium hydrogen carbonate proved to be the superior base for azetidine formation, providing 2a as the sole product with no detectable starting material or by-products (Table 1, entry 7), the use of no base at all (Table 1, entry 1) permits the formation of the desired azetidine product in a modest 44% yield. Use of lithium carbonate as base improves the conversion of amine 1a to azetidine 2a (Table 1, entry 2) a little (75%). Lithium, sodium and potassium hydroxide (Table 1, entries 6, 8 and 9 respectively) gave 50–100% conversion of amine 1a to mixtures of azetidine (2a) and pyrrolidine (3a), as did lithium and sodium acetate (Table 1, entries 10 and 11 respectively). Potassium acetate (Table 1, entry 12) showed evidence of oxidation of 1a with a product distribution that contained 50% of the desired azetidine 2a, 20% of the corresponding pyrrolidine 3a accompanied by imine 4a (30%). Potassium carbonate (Table 1, entry 4) gave complete conversion of 1a to a mixture of azetidine 2a and imine 4a (∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio). Somewhat surprisingly cesium carbonate gave only imine containing products, both the aforementioned oxidation product 4a (from oxidation of 1a) but also imine 5a (the condensation product of benzaldehyde and benzylamine).12,13 After confirming sodium hydrogen carbonate was the most effective base for azetidine formation THF, methanol, dichloromethane and DMSO (Table 1, entries 13–16 respectively) were tried as solvent. Whilst none of the solvents tried proved to be superior to acetonitrile in azetidine formation, it is noteworthy that both methanol and DMSO did show evidence of amine oxidation to imine 4a (∼30% in both cases).
2 ratio). Somewhat surprisingly cesium carbonate gave only imine containing products, both the aforementioned oxidation product 4a (from oxidation of 1a) but also imine 5a (the condensation product of benzaldehyde and benzylamine).12,13 After confirming sodium hydrogen carbonate was the most effective base for azetidine formation THF, methanol, dichloromethane and DMSO (Table 1, entries 13–16 respectively) were tried as solvent. Whilst none of the solvents tried proved to be superior to acetonitrile in azetidine formation, it is noteworthy that both methanol and DMSO did show evidence of amine oxidation to imine 4a (∼30% in both cases).
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | Base | Solvent | 1a | 2a | 3a | 4a | 5a |
| a Ratio determined by comparison of 1H NMR integrations in post work-up NMR spectra. | |||||||
| 1 | — | CH3CN | 56.0 | 44.0 | — | — | — |
| 2 | Li2CO3 | CH3CN | 25.0 | 75.0 | — | — | — |
| 3 | Na2CO3 | CH3CN | — | 61.0 | 39.0 | — | — |
| 4 | K2CO3 | CH3CN | — | 35.5 | — | 64.5 | — |
| 5 | Cs2CO3 | CH3CN | — | — | — | 82 | 18 |
| 6 | LiOH | CH3CN | 48.5 | 33.0 | 18.5 | — | — |
| 7 | NaHCO3 | CH3CN | — | >99.5 | — | — | — |
| 8 | NaOH | CH3CN | — | 60.0 | 40.0 | — | — |
| 9 | KOH | CH3CN | — | 53.0 | 47.0 | — | — |
| 10 | LiOAc | CH3CN | — | 73.0 | 27.0 | — | — |
| 11 | NaOAc | CH3CN | — | 68.5 | 31.5 | — | — |
| 12 | KOAc | CH3CN | — | 50.0 | 20.0 | 30.0 | — |
| 13 | NaHCO3 | THF | 63.0 | 37.0 | — | — | — |
| 14 | NaHCO3 | MeOH | — | 50.0 | 21.0 | 29.0 | — |
| 15 | NaHCO3 | CH2Cl2 | 58.0 | 42.0 | — | — | — |
| 16 | NaHCO3 | DMSO | — | 12.0 | 55.0 | 33.0 | — |
Next substrate scope was investigated, Table 2 shows the effect of varying the substituents labelled R1 and R2 in starting amines 1a–t. Under conditions optimised for iodoazetidine formation conversion of starting amine to cyclised products was generally high and showed no evidence for trans substitution about the azetidine (or pyrrolidine where detected) rings, the ratio of azetidine to pyrrolidine was good to excellent for benzylic R2 substituents. Aromatic substituents bearing electron donating groups in R1 (e.g. 3- and 4-OMe, Table 2, entries 9 and 17 respectively) were employed, and whilst the 3-methoxy derivative gave complete conversion to a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the corresponding azetidine and pyrrolidine the 4-methoxy derivative failed to give any cyclised product, rather an apparent loss of the allyl group and oxidation of 1r was observed. Perhaps, after initial activation of the alkene by iodine, allyl iodide might be expelled assisted by the resonance stabilization of the generated cation, as proposed in Scheme 2. Electron withdrawing groups in R1 4- and 2-nitro phenyl (Table 2, entries 6 and 13) gave 3
1 mixture of the corresponding azetidine and pyrrolidine the 4-methoxy derivative failed to give any cyclised product, rather an apparent loss of the allyl group and oxidation of 1r was observed. Perhaps, after initial activation of the alkene by iodine, allyl iodide might be expelled assisted by the resonance stabilization of the generated cation, as proposed in Scheme 2. Electron withdrawing groups in R1 4- and 2-nitro phenyl (Table 2, entries 6 and 13) gave 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of azetidine to pyrrolidine. Heteroaromatic 3- and 4-pyridyl and 3-furyl (Table 2, entries 4, 5 and 10) gave complete conversion to cyclised product with good to excellent azetidine to pyrrolidine ratios.
1 mixtures of azetidine to pyrrolidine. Heteroaromatic 3- and 4-pyridyl and 3-furyl (Table 2, entries 4, 5 and 10) gave complete conversion to cyclised product with good to excellent azetidine to pyrrolidine ratios.
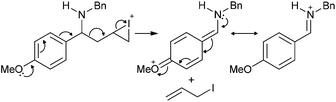 | ||
| Scheme 2 Proposed mechanism for the deallylation and imine formation observed in the reaction of 1r under typical cyclisation conditions. | ||
|
|
|||||
|---|---|---|---|---|---|
| Entry | R1 | R2 | 2 + 3/%a | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b 3b |
|
PMB = para-methoxybenzyl. PTB = para-tolylbenzyl. PNP = para-nitro-phenyl. PNB = para-nitro-benzyl. ONP = ortho-nitrophenyl. Fc = ferrocenyl.a 2 + 3 by 1H NMR spectroscopy.b cis-(2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) Ratio obtained by inspection of the 1H NMR spectrum post aqueous work-up.c Sodium carbonate was used as base.d 1r suffered loss of allyl group and oxidation to corresponding imine. 3) Ratio obtained by inspection of the 1H NMR spectrum post aqueous work-up.c Sodium carbonate was used as base.d 1r suffered loss of allyl group and oxidation to corresponding imine. |
|||||
| 1 | a | Ph | Bn | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | b | Ph | PMB | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | c | Ph | PTB | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | d | 3-Py | Bn | >99 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | e | 4-Py | Bn | >99 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | f | PNP | Bn | >99 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | g | 2-Br-Ph | Bn | >99 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | h | t-Bu | Bn | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | i | 3-OMe-Ph | Bn | >99 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | j | 3-Furyl | Bn | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | k | Cy | Bn | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | l | Ph | PNB | >99 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 13 | m | ONP | PMB | >99 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 | n | Fc | Bn | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 15 | o | Ph | Cy | 38 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >99c >99c |
| 16 | p | Ph | n-Pr | 33 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) >99c >99c |
| 17 | qd | 4-OMe-Ph | Bn | — | — |
| 18 | r | 1-Naph | Bn | 80 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 19 | s | Ph | 3-Py | >99 | >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Alkyl tert-butyl and cyclohexyl groups at R1 (Table 2, entries 8 and 11) gave complete conversion to azetidine products. An organometallic fragment in R1 ferrocenyl (Table 2, entry 14) also gave only azetidine product in quantitative consumption of homoallylamine starting material. In the variation of R2para-methoxybenzyl, para-tolylbenzyl, para-nitrobenzyl and CH2-3-pyridyl (Table 2, entries 2, 3, 12 and 20) respectively all gave quantitative conversion with only the para-nitrobenzyl derivative providing anything less than complete selectivity in favour of azetidine products. Alkyl groups in R2 proved troublesome cyclohexyl and n-propyl (Table 2, entries 15 and 16) gave low conversion to pyrrolidine products (with sodium carbonate as base), and CH2C(CH3)3 gave slightly higher conversion but the product distribution was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of pyrrolidine and azetidine (under standard conditions).
1 mixture of pyrrolidine and azetidine (under standard conditions).
The observation that the iodine mediated cyclisation consistently delivers cis relative stereochemistry can be ascribed to the suggested differences in transition state geometries that would be required to deliver cis or trans products. Addition of iodine to the carbon–carbon double bond of a homoallyl amine, such as 1a, could form two diastereomeric iodiranium species (Scheme 3). In order for cyclisation to a four-membered azetidine to occur two possible transition states are possible. The most disfavoured of the two transition states would increase a pseudo 1,3-diaxial interaction, whereas the transition state reasoned to be more favoured positions protons in the pseudo axial positions (Scheme 3: upper and lower transition state respectively).
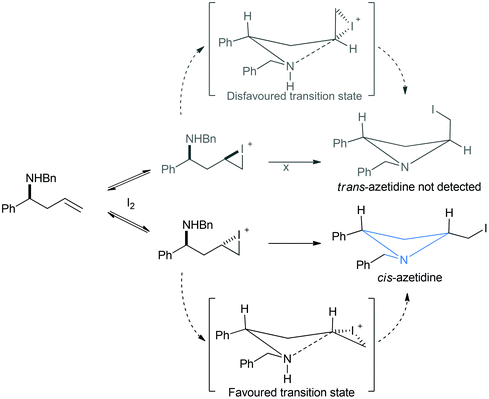 | ||
| Scheme 3 Proposed transition state argument for the cis-selectivity observed in the iodine mediated cyclisation of homoallyl amines. | ||
Since it had been already demonstrated that iodoazetidines of the general class cis-2 were highly prone to isomerisation and that the trick of rapidly reacting the crude, post work-up azetidine with a nucleophile to replace iodine could deliver stable cis-azetidine derivatives (cis-6), a range of amine derivatives of cis-azetidines 2 were prepared in 50 to 87% isolated yield over two steps from readily accessible homoallyl amines 1, Table 3. It was vital that liquid amines were used neat (as solvent) in the iodine displacement step, when co-solvents were used isomerisation to the corresponding pyrrolidines (3) became problematic, however this feature can be exploited (as discussed later) in pyrrolidine synthesis. Yields of amine bearing azetidines 6 ranged from 50 to 82% over two steps from starting homoallyl amines 1. Primary and secondary amines could be used in the derivatisation, linear and branch aliphatic, benzylic, and heteroatom containing substituents in the derivatising amine part could all be accommodated.
|
|
|---|

|
The nucleophilic displacement of iodine from cis-iodoazetidines was employed in an azide to triazole so-called click reaction sequence. Homoallyl amines 1a and 1d were reacted under standard cyclisation conditions and worked-up as before, the iodoazetidines 2a and 2d were treated with sodium azide in DMF at room temperature and stirred overnight (16 h), all the while ensuring the temperature did not exceed 20 °C, the reactions were monitored by TLC (silica EtOAc–Hex), which indicated that although the displacement of iodide by azide was incomplete the iodides 2a and 2b were showing evidence of a small level of isomerisation to the corresponding pyrrolidines (as judged by TLC). In order to minimise contamination from products of reaction after azetidine isomerisation a terminal alkyne (phenyl acetylene or pent-1-yne) was added at that point with copper(I) iodide and the reaction stirred for a further 24 h. Triazole appended azetidines 7aa, 7ab and 7da were obtained in approximately 50% yield over the three synthetic steps from homoallyl amines 1a or 1d (Scheme 4).
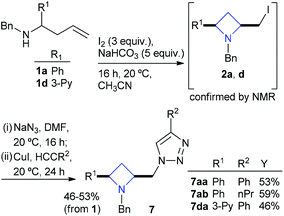 | ||
| Scheme 4 Synthesis of triazole appended azetidines 7 in ∼50% yield over three steps. | ||
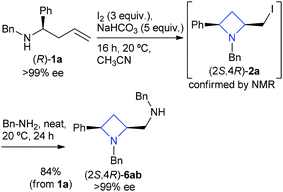 | ||
| Scheme 5 Non-racemic synthesis of amino azetidine (2S,4R)-6ab in 84% yield. | ||
The cyclisation, iodide displacement sequence was applied to the synthesis of single enantiomer azetidines (S,R)-6ab, (S,R)-6tb, (S,R)-6ua and (S,R)-6ue.14 The synthesis of (S,R)-6ab is shown in Scheme 1 which describes the use of single enantiomer 1a, no loss of enantiopurity is witnessed and (S,R)-6ab was isolated in 84% yield. Single enantiomer 1a was obtained utilising a stereoselective allylation of the corresponding imine15via an Ellman auxiliary protocol (Scheme 5).16–19
Pyrrolidines
Whilst azetidines represent an attractive synthetic target pyrrolidines, the side product of our azetidine formation protocol, are highly desirable heterocyclic constructs in the organic synthesis arena.20–23 It was shown that pyrrolidine cis-3a could be quantitatively formed upon reaction of homoallyl amines with iodine and a base with mild heating. It was also previously shown (by us in an earlier report11) that iodine containing azetidine derivative 2d stereospecifically isomerized, with time, to the corresponding pyrrolidine cis-3d. In order to confirm isomerisation of iodoazetidines to iodopyrrolidines, azetidines 2c–e were separately heated at 50 °C in acetonitrile for 4 hours (Scheme 6), in each case ≥95% of the corresponding pyrrolidine 3c–e was isolated. In this report evidence of a dissociative isomerisation mechanism is revealed, which corroborates hypotheses of Couty and others in the area (see later).24,25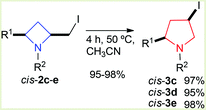 | ||
| Scheme 6 Thermal isomerisation of cis-azetidines 2c–e to cis-pyrrolidines 3c–e. | ||
Paulmier, Outurquin and co-workers reported selenium induced cyclisation of homoallyl amines to azetidines and pyrrolidines.26,27 Bott et al. reported a one carbon ring expansion of azetidines via ammonium ylide shifts.28 Couty and coworkers have studied a number of ring enlargement reactions that deliver pyrrolidines from azetidines, such as fluoride,29 chloride25 and bromide derivatives,30 alkene derivatives31 and hydroxy derivatives.25 Van Brabandt et al. also utilised ring expanded chloride and bromide bearing azetidines.32 Amongst this catalogue of work Couty and coworkers reported a computational study where ring expansion of 2-(chloromethyl)-1-isopropylazetidine was discussed,24 and noted that a polar solvent (DMSO) reduced the barrier to isomerisation and the reaction proceeds via two transition states through a strained intermediate, Scheme 7.
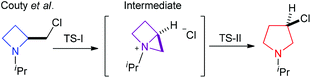 | ||
| Scheme 7 Computationally determined reaction sequence via a strained intermediate of Couty et al.24 | ||
It is also important to point out that a five (pyrrolidine) to six (piperidine) membered ring expansion reaction that exploits the nucleophilic displacement of a beta iodide by Davies et al. supports an ion pair aziridinium salt hypothesis since they were able to isolate (and characterise by single crystal X-ray diffraction) the tetrafluoroborate salt 13 of their intermediate (Scheme 8), which further reacts with acetate to give a 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 mixture of compounds 14
45 mixture of compounds 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15.33
15.33
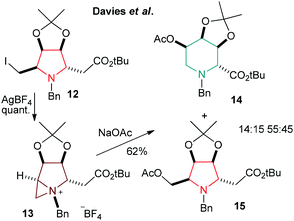 | ||
| Scheme 8 Ring expansion of 12 through aziridinium formation (13) to piperidine 14 by Davies et al.33 | ||
Using the knowledge that the isomerisation of 2,4-cis-azetidines 2 to 2,4-cis-pyrrolidines 3 is facile and our knowledge that nucleophilicly displacing iodine from azetidine products delivers stable amino azetidines we chose to run a similar protocol for generation of trans-pyrrolidine (whilst never observed it was speculated that iodopyrrolidines might be prone to polymerisation via N-quaternisation or other side reactions so reaction of the iodide directly was deemed appropriate). It was envisaged that, in one pot, a homoallyl amine (1) could be cyclised to a 2,4-cis-azetidine (2), which in turn could be isomerised by action of heat to a 2,4-cis-pyrrolidine (3). The cis-pyrrolidine thus formed upon treatment with a nucleophile would deliver a trans-substituted pyrrolidine. Indeed, for the case of homoallyl amines 1a, c, d and m the corresponding cis-azetidines (2a, c, d and m) and then cis-pyrrolidines (3a, c, d and m) were identified by proton NMR spectroscopy and treated with a primary amine to give 2,4-trans-pyrrolidines 8ab, aj and kb (>73% yield over three steps) or with sodium azide followed by a terminal alkyne and a copper(I) catalyst to give 2,4-trans-pyrrolidines 9aa, ca and da as single diastereoisomers in good isolated yields (57–65% yield over four steps), Scheme 9 – left side. An X-ray crystal structure of trans-9da helped confirm the relative trans-stereochemistry (inset Scheme 9). To our surprise (and later delight) when cis-azetidines 2a and 2k (confirmed by proton NMR spectroscopy) where stirred at room temperature in DMSO with primary amine nucleophiles not only were pyrrolidines the major products (as indicated earlier in the azetidine section, use of a solvent rather than neat amine gave some pyrrolidine product) but these products were the cis-diastereoisomer 8ab, aj and kb in greater than 77% isolated yield from the corresponding homoallylamines, Scheme 9 – right side. Thus it is possible to synthesise both diastereoisomers of the 2,4-pyrrolidines from the same starting material in good yield and high diastereoselectivity simply by adjusting the addition sequence and reaction temperature. Alongside this finding it was also possible to prepare cis-2,4-hydroxy functionalised pyrrolidines (although it was not possible to obtain the trans isomer) by using hydroxide as the nucleophile in 72–89% yield in the cases tried (including a pyrrolidine bearing a 3-pyridyl functionality in R1 offering access to functionalised analogues of a nicotine core).
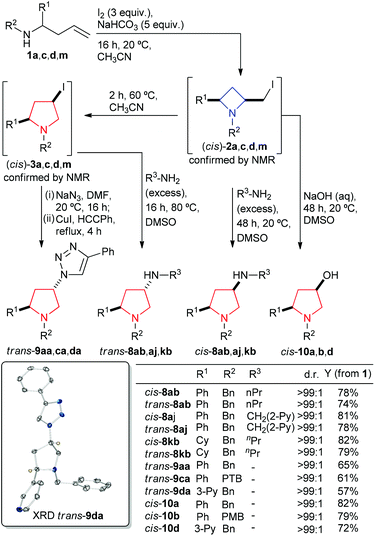 | ||
| Scheme 9 The synthesis of 2,4-cis- and trans-pyrrolidines (8, 9 and 10) from homoallyl amines (1). Inset: X-ray crystal structure of trans-9da, only pyrrolidine and triazole C–H protons shown for clarity, nitrogen atoms are coloured blue (Ortep plot 30% probability ellipsoids), for full details see ESI.† | ||
The observation that both cis- and trans-pyrrolidines 8 are readily accessible from the same starting materials by simply subtly changing the reaction conditions (namely adding nucleophile before or after isomerisation of azetidine to pyrrolidine) leads to the conclusion that the isomerisation is likely to proceed via intermediate 11, Scheme 10. In the absence of any external nucleophile the cis-azetidine isomerises to the corresponding cis-pyrrolidines 3, which can then undergo an SN2 reaction with a nucleophile, such as a primary amine, to give trans-substituted pyrrolidines 8. However, intermediate 11 ought to be stabilised by polar solvent (according to calculations for a related system by Couty et al.), so if it forms in the presence of an external nucleophile (e.g. primary amine) it is intercepted to give cis-8.
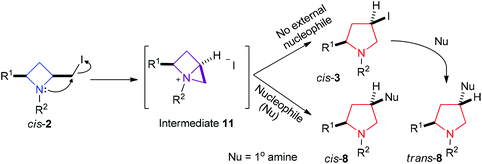 | ||
| Scheme 10 Divergent conversion of cis-azetidines 2via intermediate 11 to both cis- and trans-pyrrolidines 8. | ||
Preliminary biological activity
Zebrafish embryo development can be used as an assay to assess potential drug-like behaviour of molecules in a complex organism; certain developmental manifestations can help to provide early indications of possible utility in therapeutic regimes and also toxic side-effects that could develop.34–41Zebrafish are a suitable vertebrate model for drug screening due to their ease of access, low maintenance costs, optical transparency, fast development and relatedness to humans in terms of physiology, genetics and toxic responses.40 In comparison, rodent models are more expensive to maintain, their development is slow and occurs in utero making drug testing difficult and the number of embryos that can be obtained is also small reducing the scale of the experiments.42 Additionally, in comparison to in vitro cell culture models, the zebrafish embryo enables the response of the organism as a whole to a compound to be observed, such as cell–cell and cell–matrix interactions, as well as enabling the efficiency of uptake, metabolism and excretion of a compound to be determined; information that cannot be obtained from in vitro cell culture models.42 Zebrafish screens require small amounts of compound, which saves money and the compound can also be added directly to the bathing solution, so injection is not required for drug delivery. Zebrafish screens can also be automated enabling high throughput screening to be carried out where thousands of compounds can be tested at one time. The first chemical screen in zebrafish was carried out by Peterson and colleagues in 2000 where compounds from a library were screened in zebrafish to see what phenotypes they evoked, some of the phenotypes observed included effects on pigmentation and ear development.43 These phenotype based screens can help to uncover the mechanism by which compounds may be acting. For example when fertilised zebrafish embryos are exposed to a certain concentration of probe molecule and the morphological changes are monitored, reduced pigmentation can be an indicator that the probe molecule may be acting on thyroid hormone signaling and therefore warrants further investigation as an agent for treating thyroid disorders.44 In a forward chemical screen in zebrafish carried out by Das et al. a retinoid analogue which caused specific cardiovascular defects in zebrafish (heart tube deformations) was identified, indicating potential usefulness against conditions such as acute promyelocytic leukaemia.45
For our investigation of biological activity using zebrafish embryo development as a screening technique six azetidine derivatives were selected. The azetidines selected, racemic cis-6ae, 6ac, 6ad, 6ja, 6ga and 6di represent a cross section of the azetidines synthesized and provide an interesting comparison in terms of the chemical space they explore, Fig. 2. Comparisons between the structures selected could be made along the following lines tertiary amine versus secondary amine; aliphatic versus benzylic; heteroaromatic versus aromatic; etc.
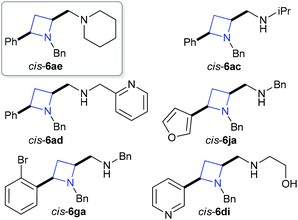 | ||
| Fig. 2 Azetidines selected for preliminary biological screening. | ||
Zebrafish embryo developmental morphology assay
Table 4 shows a summary of the morphological defects observed after five days following treatment of zebrafish embryos at 75%-epiboly stage with the six selected azetidines at a concentration of 25 μM. Azetidines were added as DMSO solutions against a control and a DMSO blank. Most compounds had minimal effects on overall morphology (see Table S1, ESI†). However, compound cis-6ae was found to cause severe morphological effects over the whole exposure period showing increased severity of phenotypes at higher doses (see Fig. S1 to S8, ESI†), cis-6ga was also found to cause morphological effects at later time points (see Fig. S9, ESI†).| Morphological defects | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Additive | Curved tails | Abnormal jaw | Delayed growth | Lack of pigmentation | Slowed heart rate | Tail necrosis | Cardiac oedema | Brain oedema | Reduced circulation/blood clots |
| ++++ = very severe effect (75–100%); +++ = severe effect (50–75%); ++ = moderate effect (25–50%); + = minimal effect (5–25%); +/− = either minimal or no effect (0–5%); − = no effect (0%). | |||||||||
| cis-6ae | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ | +++ | ++++ |
| cis-6ja | + | — | + | — | ++ | — | ++ | — | ++ |
| cis-6ga | +++ | — | + | — | +++ | — | +++ | — | +++ |
| cis-6ac | + | — | +/− | — | + | — | + | — | + |
| cis-6di | +/− | — | — | — | +/− | — | +/− | — | +/− |
| cis-6ad | +/− | +/− | +/− | — | + | — | + | — | + |
| Control | +/− | — | +/− | — | +/− | — | +/− | — | +/− |
| Blank | — | — | — | — | +/− | — | +/− | — | +/− |
Compound cis-6ae, which contains a piperidine fragment, shows effects in all of the nine morphological defect indicators. This suggests cis-6ae has high biological activity in a number of tissues, resembling many effects seen when zebrafish embryos are exposed to piperidines, effects such as hypopigmentation and cardiac edema.46 Next most active is compound cis-6ga, which bears an ortho-bromophenyl substituent in the 2-position of the of the azetidine ring. Compound cis-6ga resulted in curved tails, slowed heart rate, cardiac edema and reduced circulation in more than 50% of embryos. The 3-furyl derivative 6ja showed a similar but less extensive spread of defects. Compounds cis-6ae, cis-6ga and cis-6ja all produced effects similar to those seen with nicotine;47 however, they showed varying degrees of severity. An additional feature observed with cis-6ae not included in the morphological defect scoring was the development of an atrioventricular block, specifically a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 type (data not shown), which is often observed when zebrafish embryos are treated with QT prolonging drugs,48 suggesting that this compound in particular may also be affecting cardiac ion channels. Additionally, somites were found to be u-shaped and melanophores were found to be small and granulated, as well as reduced in number. U-shaped somites are characteristic features observed when there are defects in slow muscle development caused by disruption to the Hedgehog signaling pathway.49 The pigmentation effects seen may have been caused by reduced cyclic adenosine monophosphate (cAMP) levels and disruption of microtubule function, as both are required for aggregating and dispersing melanin.50 Additional disruption to xanthophores may have also contributed to the pigmentation phenotype at later stages.51 Embryos treated with compound cis-6ae were also unable to hatch, which could have been due to the hatching enzyme chorionase being inhibited, osmotic disturbances or due to weakened muscular movements.52 The other compounds tested in this preliminary screen did not show any marked defects, which could be due to the small concentration range tested, as different compounds are likely to have different effective concentrations. However, it is clear that among even this small sample set various important pharmacological effects can be seen and small structure variation have wide impact on activity, boding well for future investigations. Since these compounds have not been previously tested in zebrafish, correlation to a similar molecule can provide important benchmarking, a small ring nitrogen-containing heterocycle which has been extensively studied is nicotine, which is a naturally occurring alkaloid that acts on nACh receptors. Nicotine in is extremely toxic to humans and is a known human teratogen.53 In zebrafish embryos, nicotine has been found to reduce notochord length, cause apoptotic death of brain cells,54 lead to underdeveloped tails, reduced number of somites, increased muscular bends in spine and cause complications in jaw formation.53 Many of these effects correlate with those seen with some of the azetidines screened here, particularly compound cis-6ae. The muscular bends, which are common features of exposure to compound cis-6ae and cis-6ga are thought to occur due to slow muscle fibres becoming disorganised and the nACh receptors becoming desensitised, which explains the short stature of the fish and the bent tails.55 With compound cis-6ae reduced locomotion was observed when 6 dpf larval zebrafish were treated at a concentration of 20 μM, which is similar to that which occurs when zebrafish are treated with high concentrations of nicotine.56 This is because at low concentrations, nicotine causes stimulation of nACh receptors in the CNS, whereas at higher concentrations nicotine will bind more preferentially to nACh receptors in muscles (particularly those involved in movement), which can have negative depressant effects reducing locomotion.
1 type (data not shown), which is often observed when zebrafish embryos are treated with QT prolonging drugs,48 suggesting that this compound in particular may also be affecting cardiac ion channels. Additionally, somites were found to be u-shaped and melanophores were found to be small and granulated, as well as reduced in number. U-shaped somites are characteristic features observed when there are defects in slow muscle development caused by disruption to the Hedgehog signaling pathway.49 The pigmentation effects seen may have been caused by reduced cyclic adenosine monophosphate (cAMP) levels and disruption of microtubule function, as both are required for aggregating and dispersing melanin.50 Additional disruption to xanthophores may have also contributed to the pigmentation phenotype at later stages.51 Embryos treated with compound cis-6ae were also unable to hatch, which could have been due to the hatching enzyme chorionase being inhibited, osmotic disturbances or due to weakened muscular movements.52 The other compounds tested in this preliminary screen did not show any marked defects, which could be due to the small concentration range tested, as different compounds are likely to have different effective concentrations. However, it is clear that among even this small sample set various important pharmacological effects can be seen and small structure variation have wide impact on activity, boding well for future investigations. Since these compounds have not been previously tested in zebrafish, correlation to a similar molecule can provide important benchmarking, a small ring nitrogen-containing heterocycle which has been extensively studied is nicotine, which is a naturally occurring alkaloid that acts on nACh receptors. Nicotine in is extremely toxic to humans and is a known human teratogen.53 In zebrafish embryos, nicotine has been found to reduce notochord length, cause apoptotic death of brain cells,54 lead to underdeveloped tails, reduced number of somites, increased muscular bends in spine and cause complications in jaw formation.53 Many of these effects correlate with those seen with some of the azetidines screened here, particularly compound cis-6ae. The muscular bends, which are common features of exposure to compound cis-6ae and cis-6ga are thought to occur due to slow muscle fibres becoming disorganised and the nACh receptors becoming desensitised, which explains the short stature of the fish and the bent tails.55 With compound cis-6ae reduced locomotion was observed when 6 dpf larval zebrafish were treated at a concentration of 20 μM, which is similar to that which occurs when zebrafish are treated with high concentrations of nicotine.56 This is because at low concentrations, nicotine causes stimulation of nACh receptors in the CNS, whereas at higher concentrations nicotine will bind more preferentially to nACh receptors in muscles (particularly those involved in movement), which can have negative depressant effects reducing locomotion.
Since compound cis-6ae was shown to elicit morphological defects across the nine probed criteria it was selected for a more detailed investigation. Specific characterisation (detailed phenotypic analysis) of compound 6ae to look for further effects undetectable by morphology analysis alone was embarked upon (Fig. 3). Analysis of locomotion by assessing motility of zebrafish larvae through measurement of pixel change over time, overall blood vessel development through fluorescence visualisation of vascular endothelial cells using the Tg(fli-1:EGFP) transgenic zebrafish line and blood circulation through fluorescence visualisation of red blood cells using Tg(gata-1:dsRed); Tg(fli-1:EGFP) transgenic zebrafish line revealed that compound cis-6ae reduced locomotion, did not affect gross blood vessel development and reduced circulation with formation of blood clots. Compound cis-6ae at a concentration of 20 μM was found to significantly reduce movement of 6 dpf zebrafish compared to controls (Fig. 3, A) (n = 60, P < 0.05). Morphological defects observed after 48 hours following treatment with 25 μM cis-6ae at 75%-epiboly stage included bent tails, hypopigmentation, severely delayed development, brain and cardiac edema (Fig. 3, B). Analysis of 2 dpf Tg(fli-1:EGFP) zebrafish treated at 75%-epiboly with 50 μM cis-6ae (Fig. 3, C) under the green channel (which marks blood vessels) showed severely delayed growth with the tip of the tail not fully formed and tail necrosis was evident with no blood vessels present in the necrotic part of the tail. Tail necrosis was found to occur in all embryos treated with 50 μM cis-6ae (Fig. 3, D) (n = 40, P < 0.05). However, overall vascular development was unperturbed. Analysis of 2 dpf Tg(gata-1:dsRed); Tg(fli-1:EGFP) zebrafish treated at 75%-epiboly with 50 μM cis-6ae (Fig. 3, E) under the red channel (which labels circulating blood cells) showed reduced circulation and the formation of blood clots. The formation of blood clots was found to occur in all embryos treated with 50 μM cis-6ae with aggregation of erythrocytes evident at the end of the tail due to reduced blood circulation (Fig. 3, F) (n = 40; P < 0.05).
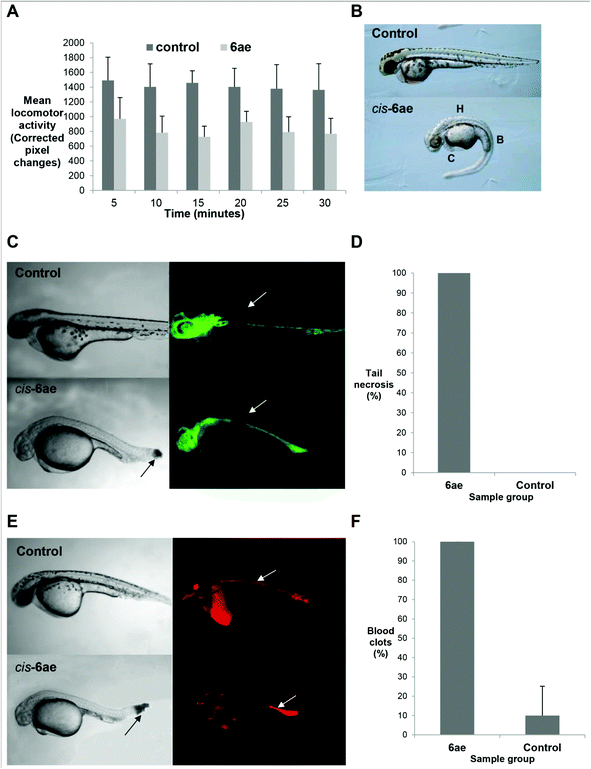 | ||
| Fig. 3 Effects of compound cis-6ae: (A) bar chart comparing movement/locomotion of cis-6ae-treated 6 dpf zebrafish larvae compared to controls; (B) bright field lateral view of a cis-6ae-treated 2 dpf embryo presenting with morphological defects (H = hypopigmentation, B = bent spine, C = cardiac edema) compared to control; (C) bright field lateral view of a cis-6ae-treated 36 hpf zebrafish embryo compared to control (left) with corresponding fluorescence images of vasculature through fli-1:EGFP transgene activity (right) where individual blood vessels can be seen (arrows) and the formation of a blood clot in the cis-6ae-treated embryo; (D) bar chart comparing frequency of tail necrosis in cis-6ae-treated 36 hpf embryos compared to controls; (E) bright field lateral view of a cis-6ae-treated 36 hpf zebrafish embryo compared to control (left) with corresponding fluorescence images of blood circulation through gata-1:dsRed transgene activity (right) where erythrocytes can be seen (arrows) and the formation of a blood clot in the cis-6ae-treated embryo; (F) bar chart comparing frequency of blood clots in cis-6ae-treated 36 hpf embryos compared to controls. | ||
Exposure to compound cis-6ae resulted in numerous morphological defects in zebrafish embryos, suggesting that it was able to act on many tissues. Many similarities to nicotine toxicity were also observed suggesting that it may have a similar mode of action or operate via a similar route. Compound cis-6ae displayed similar effects to those which have been documented in zebrafish embryos following treatment with different piperidines, such as 2-methylpiperidine,46 such compounds are often used as insecticides.57 Recent studies with pyrethroid insecticides also showed similar morphological defects, such as jaw abnormalities, curvature of body axis and pericardial edema.58 Additionally, the pigmentation defects observed with cis-6ae are similar to those seen with anti-thyroid agents such as methimazole,59 suggesting there might be an effect on thyroid hormone signalling. However, there are many ways in which pigmentation can be reduced (e.g. disturbances to non-skeletal neural crest or melanophore formation), therefore further investigation would be required to ascertain the precise cause in this case. Overall, cis-6ae shows high toxicity at the concentrations that were used in this study and would be detrimental particularly to aquatic habitats if it were to be released into such an environment. Further studies to investigate mechanism of action and dose ranging may reveal potential therapeutic benefits (e.g. treatment for overactive thyroid/lice/scabies) or industrial applications (e.g. use as an agricultural insecticide).
From the selection of compounds tested at the specified concentrations, compound cis-6ae, as mentioned previously, has detrimental effects on zebrafish embryo development, making this compound particularly toxic to aquatic environments. Further studies would be necessary to elucidate its mechanism of action and metabolism in zebrafish. This study however shows that the zebrafish embryo is a suitable vertebrate model to investigate lead compounds for toxic effects due to its ease of accessibility, optical transparency, contribution to 3Rs (replacement, reduction and refinement) of animals in research, fast development, low cost of maintenance and high similarity in toxic responses when compared to humans and rodents. These advantages make the zebrafish embryo an attractive model for testing during pre-clinical drug development before tests are performed in rodents or higher mammals, saving time and money by filtering out any lead compounds with safety liabilities before they reach the next step in the drug development pipeline.
Conclusions
A synthetically versatile protocol for the stereospecific cyclisation of homoallyl amine derivatives ultimately delivered cis-azetidine and cis or trans pyrrolidine derivatives. Importantly, it was the subtle change of temperature and reaction sequence in this iodine and base mediated cyclisation and subsequent halide displacement that gave selective access to these three groups of compound. A zebrafish embryo developmental morphology assay was demonstrated as a useful tool in assessing the biological activity of a sample of azetidine derivatives and a compound bearing an azetidine and a piperidine to elicit morphological responses across the panel of indicators of potential activity in a variety of settings. This demonstrates both the utility of the fertilised zebrafish egg morphology screening technique and the potential for the underlying azetidine scaffold discussed to be probed for biological activity using advanced high throughput techniques employing zebrafish embryos. With the increasing number of transgenic lines available and the latest technological advances the zebrafish will provide a platform for drug screening in the years to come.Acknowledgements
The authors thank the University of Birmingham and ERDF AWM II for support. AF thanks The School of Chemistry, University of Birmingham for a studentship and the EPSRC for underpinning support. MAHS thanks the HCDP - Ministry of Higher Education-Kurdistan Region for financial support. JSF is extremely grateful to the Leverhulme Trust (F/00351/P and F/00094/BC) for early funding, East China University of Science and Technology for a visiting professorship, the Natural Science Foundation of China for a research fellowship (no. 21050110426) and the Royal Society Industry Fellowship scheme (2012/R1) for on-going support. FM and SSD thank the Marie Curie ITN project BOLD (Biology of liver and pancreatic development and disease) and “ZF-Health” Integrating projects in the Framework 7 programme of the European Commission for their financial support. The EPSRC UK National Crystallography Service at the University of Southampton are thanked for the collection of the crystallographic data.60References
-
G. Cardillo, L. Gentilucci and A. Tolomelli, in Asymmetric Synthesis of Nitrogen Heterocycles, ed. J. Royer, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, pp. 1–50 Search PubMed
.
- J. F. Ma and K. Nomoto, Plant Physiol., 1993, 102, 373–378 CAS
.
- T. Takemoto, K. Nomoto, S. Fushiya, R. Ouchi, G. Kusano, H. Hikino, S. I. Takagi, Y. Matsuura and M. Kakudo, Proc. Jpn. Acad., Ser. B, 1978, 54, 469–473 CrossRef CAS
.
- S. Knapp and Y. H. Dong, Tetrahedron Lett., 1997, 38, 3813–3816 CrossRef CAS
.
- H. Takikawa, T. Maeda and K. Mori, Tetrahedron Lett., 1995, 36, 7689–7692 CrossRef CAS
.
- H. Yoda, T. Uemura and K. Takabe, Tetrahedron Lett., 2003, 44, 977–979 CrossRef CAS
.
- J. D. Schmitt, Curr. Med. Chem., 2000, 7, 749–800 CrossRef CAS
.
- D. X. Wang, H. Booth, N. Lerner-Marmarosh, T. S. Osdene and L. G. Abood, Drug Dev. Res., 1998, 45, 10–16 CrossRef CAS
.
- J. T. Lowe, M. D. Lee, L. B. Akella, E. Davoine, E. J. Donckele, L. Durak, J. R. Duvall, B. Gerard, E. B. Holson, A. Joliton, S. Kesavan, B. C. Lemercier, H. Liu, J.-C. Marié, C. A. Mulrooney, G. Muncipinto, M. Welzel-O'Shea, L. M. Panko, A. Rowley, B.-C. Suh, M. Thomas, F. F. Wagner, J. Wei, M. A. Foley and L. A. Marcaurelle, J. Org. Chem., 2012, 77, 7187–7211 CrossRef CAS
.
- J. Frigola, J. Pares, J. Corbera, D. Vano, R. Merce, A. Torrens, J. Mas and E. Valenti, J. Med. Chem., 1993, 36, 801–810 CrossRef CAS
.
- A. Feula, L. Male and J. S. Fossey, Org. Lett., 2010, 12, 5044–5047 CrossRef CAS
.
- The unexpected oxidation, deallylation, sequence witnessed when using cesium carbonate as base was examined in detail in a parallel report, see next reference.
- A. Feula and J. S. Fossey, RSC Adv., 2013, 3, 5370 RSC
.
- See ESI† for details.
- S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS
.
- G. C. Liu, D. A. Cogan and J. A. Ellman, J. Am. Chem. Soc., 1997, 119, 9913–9914 CrossRef CAS
.
- G. C. Liu, D. A. Cogan, T. D. Owens, T. P. Tang and J. A. Ellman, J. Org. Chem., 1999, 64, 1278–1284 CrossRef CAS
.
- D. A. Cogan, G. C. Liu and J. Ellman, Tetrahedron, 1999, 55, 8883–8904 CrossRef CAS
.
- J. A. Ellman, T. D. Owens and T. P. Tang, Acc. Chem. Res., 2002, 35, 984–995 CrossRef CAS
.
- T. Harrison, Contemp. Org. Synth., 1995, 2, 209–224 RSC
.
- M. Wang, Z. Wang, Y.-H. Shi, X.-X. Shi, J. S. Fossey and W.-P. Deng, Angew. Chem., Int. Ed., 2011, 50, 4897–4900 CrossRef CAS
.
- M. P. Patil, A. K. Sharma and R. B. Sunoj, J. Org. Chem., 2010, 75, 7310–7321 CrossRef CAS
.
- G. Barker, J. L. McGrath, A. Klapars, D. Stead, G. Zhou, K. R. Campos and P. O'Brien, J. Org. Chem., 2011, 76, 5936–5953 CrossRef CAS
.
- F. Couty and M. Kletskii, J. Mol. Struct. (THEOCHEM), 2009, 908, 26–30 CrossRef CAS
.
- F. Durrat, M. V. Sanchez, F. Couty, G. Evano and J. Marrot, Eur. J. Org. Chem., 2008, 3286–3297 CrossRef CAS
.
- B. Berthe, F. Outurquin and C. Paulmier, Tetrahedron Lett., 1997, 38, 1393–1396 CrossRef CAS
.
- F. Outurquin, X. Pannecoucke, B. Berthe and C. Paulmier, Eur. J. Org. Chem., 2002, 1007–1014 CrossRef CAS
.
- T. M. Bott, J. A. Vanecko and F. G. West, J. Org. Chem., 2009, 74, 2832–2836 CrossRef CAS
.
- B. Drouillat, F. Couty, O. David, G. Evano and J. Marrot, Synlett, 2008, 1348 Search PubMed
.
- F. Couty, F. Durrat and D. Prim, Tetrahedron Lett., 2003, 44, 5209–5212 CrossRef CAS
.
- F. Couty, F. Durrat, G. Evano and J. Marrot, Eur. J. Org. Chem., 2006, 4214–4223 CrossRef CAS
.
- W. Van Brabandt, R. Van Landeghem and N. De Kimpe, Org. Lett., 2006, 8, 1105–1108 CrossRef CAS
.
- S. G. Davies, R. L. Nicholson, P. D. Price, P. M. Roberts, A. J. Russell, E. D. Savory, A. D. Smith and J. E. Thomson, Tetrahedron: Asymmetry, 2009, 20, 758–772 CrossRef CAS
.
- C. Delvecchio, J. Tiefenbach and H. M. Krause, Assay Drug Dev. Technol., 2011, 9, 354–361 CrossRef CAS
.
- C. A. Macrae, Expert Opin. Drug Discovery, 2010, 5, 619–632 CrossRef CAS
.
- T. V. Bowman and L. I. Zon, ACS Chem. Biol., 2010, 5, 159–161 CrossRef CAS
.
- C. Chakraborty, C. H. Hsu, Z. H. Wen, C. S. Lin and G. Agoramoorthy, Curr. Drug Metab., 2009, 10, 116–124 CrossRef CAS
.
- T. P. Barros, W. K. Alderton, H. M. Reynolds, A. G. Roach and S. Berghmans, Br. J. Pharmacol., 2008, 154, 1400–1413 CrossRef CAS
.
- L. I. Zon and R. T. Peterson, Nat. Rev. Drug Discovery, 2005, 4, 35–44 CrossRef CAS
.
- G. J. Lieschke and P. D. Currie, Nat. Rev. Genet., 2007, 8, 353–367 CrossRef CAS
.
- R. T. Peterson and M. C. Fishman, Methods Cell Biol., 2011, 105, 525–541 CrossRef CAS
.
- G. N. Wheeler and A. W. Brandli, Dev. Dyn., 2009, 238, 1287–1308 CrossRef CAS
.
- R. T. Peterson, B. A. Link, J. E. Dowling and S. L. Schreiber, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 12965–12969 CrossRef CAS
.
- O. A. Elsalini and K. B. Rohr, Dev. Genes Evol., 2003, 212, 593–598 CAS
.
- B. C. Das, K. McCartin, T. C. Liu, R. T. Peterson and T. Evans, PLoS One, 2010, 5, e10004 Search PubMed
.
- C. Schulte and R. Nagel, ATLA, Altern. Lab. Anim., 1994, 22, 12–19 Search PubMed
.
- L. T. Thomas, L. Welsh, F. Galvez and K. R. Svoboda, Toxicol. Appl. Pharmacol., 2009, 239, 1–12 CrossRef CAS
.
- U. Langheinrich, G. Vacun and T. Wagner, Toxicol. Appl. Pharmacol., 2003, 193, 370–382 CrossRef CAS
.
- I. G. Woods and W. S. Talbot, PLoS Biol., 2005, 3, e66 CrossRef
.
- D. W. Logan, S. F. Burn and I. J. Jackson, Pigm. Cell Res., 2006, 19, 206–213 CrossRef CAS
.
- J. Odenthal, K. Rossnagel, P. Haffter, R. N. Kelsh, E. Vogelsang, M. Brand, F. J. van Eeden, M. Furutani-Seiki, M. Granato, M. Hammerschmidt, C. P. Heisenberg, Y. J. Jiang, D. A. Kane, M. C. Mullins and C. Nusslein-Volhard, Development, 1996, 123, 391–398 CAS
.
- Q. He, K. Liu, S. Wang, H. Hou, Y. Yuan and X. Wang, Drug Chem. Toxicol., 2012, 35, 149–154 CrossRef CAS
.
- E. W. Klee, J. O. Ebbert, H. Schneider, R. D. Hurt and S. C. Ekker, Nicotine Tob. Res., 2011, 13, 301–312 CrossRef CAS
.
- B. Parker and V. P. Connaughton, Zebrafish, 2007, 4, 59–68 CrossRef CAS
.
- L. Welsh, R. L. Tanguay and K. R. Svoboda, Toxicol. Appl. Pharmacol., 2009, 237, 29–40 CrossRef CAS
.
- A. M. Petzold, D. Balciunas, S. Sivasubbu, K. J. Clark, V. M. Bedell, S. E. Westcot, S. R. Myers, G. L. Moulder, M. J. Thomas and S. C. Ekker, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18662–18667 CrossRef CAS
.
- K. A. Bandara, V. Kumar, U. Jacobsson and L. P. Molleyres, Phytochemistry, 2000, 54, 29–32 CrossRef CAS
.
- A. DeMicco, K. R. Cooper, J. R. Richardson and L. A. White, Toxicol. Sci., 2010, 113, 177–186 CrossRef CAS
.
- O. A. Elsalini and K. B. Rohr, Dev. Genes. Evol., 2003, 212, 593–598 CAS
.
- S. J. Coles and P. A. Gale, Chem. Sci., 2012, 3, 683–689 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: General and experimental chemical procedures, biological procedures and statistics and X-ray crystallographic information are available. CCDC 936733. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ob41007b |
| This journal is © The Royal Society of Chemistry 2013 |



