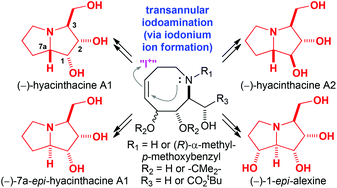
The transannular iodoamination of substituted 1,2,3,4,7,8-hexahydroazocine scaffolds has been developed into a versatile, diastereodivergent route to enable the synthesis of a range of pyrrolizidine alkaloids, as demonstrated by the syntheses of (−)-hyacinthacine A1, (−)-7a-epi-hyacinthacine A1, (−)-hyacinthacine A2, and (−)-1-epi-alexine. The requisite 1,2,3,4,7,8-hexahydroazocines (bearing either an N-α-methyl-p-methoxybenzyl group or no N-substituent) were readily prepared via conjugate addition of lithium (R)-N-but-3-enyl-N-(α-methyl-p-methoxybenzyl)amide to either tert-butyl (4S,5R,E)-4,5-dihydroxy-4,5-O-isopropylidene-2,7-dienoate (derived from D-ribose) or tert-butyl (S,S,E)-4,5-dihydroxy-4,5-O-isopropylidene-2,7-dienoate (derived from L-tartaric acid) coupled with in situ enolate oxidation with (−)-camphorsulfonyloxaziridine, followed by ring-closing metathesis with Grubbs I catalyst. Subsequent reaction with I2 resulted in transannular iodoamination (accompanied by concomitant loss of the N-α-methyl-p-methoxybenzyl group for tertiary amine substrates) to give the corresponding pyrrolizidine scaffolds.

 Please wait while we load your content...
Please wait while we load your content...