Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Triterpenoids
Robert A.
Hill
* and
Joseph D.
Connolly
School of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bob.hill@glasgow.ac.uk
First published on 5th June 2013
Abstract
Covering: 2011. Previous review: Nat. Prod. Rep., 2012, 29, 780–818.
This review covers the isolation and structure determination of triterpenoids including squalene derivatives, lanostanes, holostanes, cycloartanes, cucurbitanes, dammaranes, euphanes, tirucallanes, tetranortriterpenoids, quassinoids, lupanes, oleananes, friedelanes, ursanes, hopanes, onoceranes and saponins; 308 references are cited.
1 Introduction
Interest in the biological activities of triterpenoids continues with reviews on their anti-inflamatory,1,2 antiviral,3 antitumour,4–6 anti-HIV7 and insecticidal8 activities and for treatment of metabolic and vascular diseases.9 Surveys have appeared describing the triterpenoids isolated from Anemone raddeana,10Poria cocos,11Lantana12 and Simaba13 species and Pinaceae14 and Meliaceae15 families. Triterpenoid saponins show a range of biological activities16 and this has generated interest in their biosynthesis17 and improvement of yields from natural sources.18 Reviews covering triterpenoid saponins from Camellia19 and Polygala20 species and the Theaceae21 and Caryophyllaceae and Illecebraceae22 families have appeared.2 The squalene group
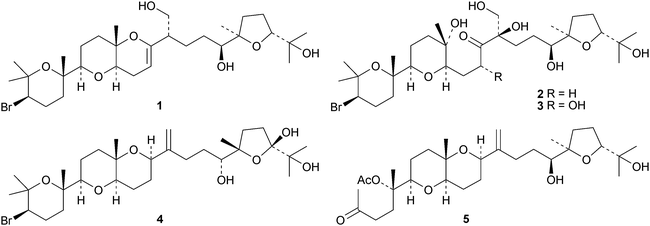
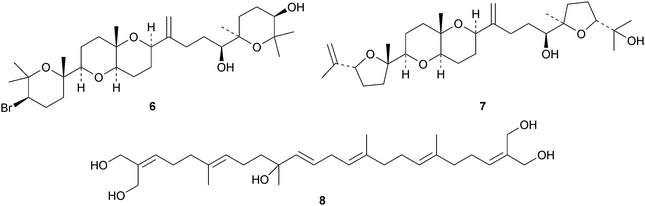
15-Dehydroxythyrsenol A 1, prethyrsenol A 2 and 13-hydroxyprethyrsenol A 3 are new cytotoxic squalene derivatives from Laurencia viridis.23 The related compounds 22-hydroxy-15(28)-dehydrovenustatriol 4, secodehydrothyrsiferol 5, iubol 6 and 1,2-dehydropseudodehydrothysiferol 7 have also been isolated from Laurencia viridis by the same group.24 Squalene-1,10,24,25,30-pentol 8, which shows moderate antimycobacterial activity, has been reported from the leaves and twigs of Rhus taitensis.25 The biochemistry and molecular biology of squalene has been reviewed.263 The lanostane group
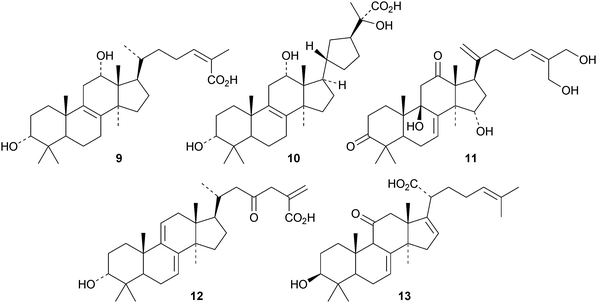
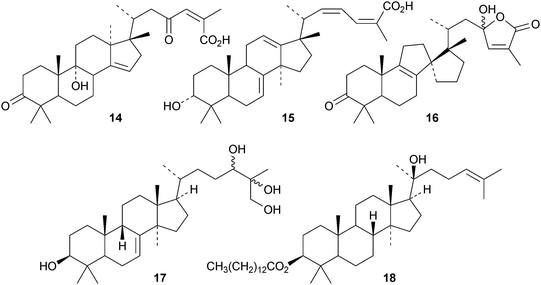
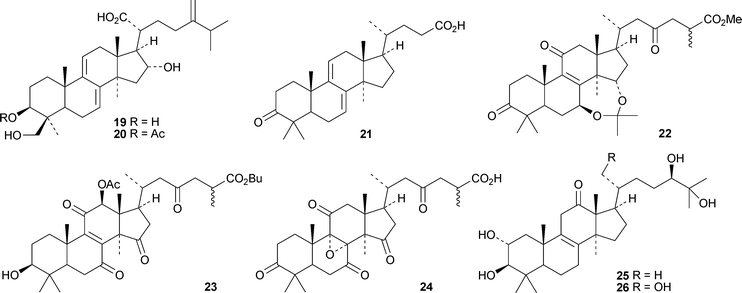
Schiglauzic acid 9 and schiglaucyclozic acid 10 are new lanostanes from the stems of Schisandra glaucescens.27 The structures of both compounds were confirmed by X-ray analyses. The three lanostanes 11, 12 and 13, from the leaves of Abies spectabilis, are accompanied by the mariesane derivative 14 and the 18(13→17)-abeo-lanostane 15.28 The rearranged lanostane 16 and 24,25,26-trihydroxylanost-7-en-3-one 17 have been isolated from Abies nephrolepis.29 The tetradecanoyl ester 18 is a constituent of Euphorbia sapinii.30Bioactive lanostane derivatives from fungi include 19 and 20 from Poria cocos31 and 24,25,26-trinor-3-oxolanosta-7,9(11)-dien-24-oic acid 2132 and methyl ganoderate A acetonide 22 and butyl ganoderate H 2333 from Ganoderma lucidum. The epoxyganoderic acid 24 is also a constituent of Ganoderma lucidum.34 The biological properties of triterpenoids from Poria cocos35 and Ganoderma lucidum36 have been reviewed.
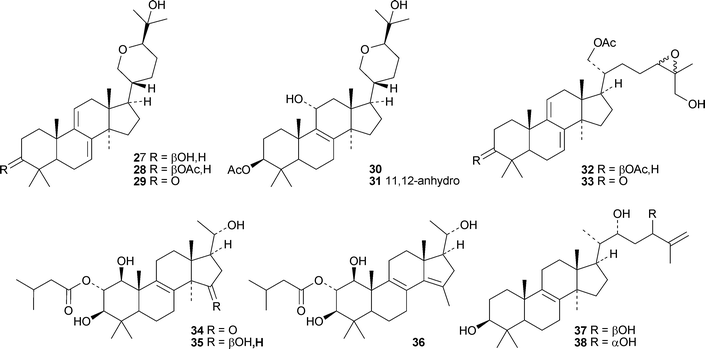
Fasciculols H 25 and I 26 are constituents of the Chinese mushroom Naematoloma fasciculare.37 The entomopathogenic fungus Hypocrella sp. BCC 14524 is the source of the lanostanes hypocrellols A–G 27–33.38 Xylariacins A 34, B 35 and C 36 have been isolated from Xylarialeum sp. A45, an endophytic fungus isolated from Annona squamosa.39 Inonotsutriols D 37 and E 38 have been reported from the white rot fungus Inonotus obliquus.40
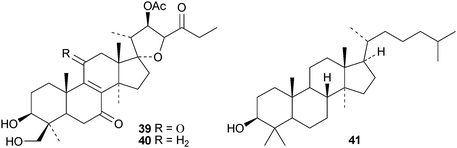
Erylosides R1, T1, T2, T3, T4, T5 and T6 are lanostanesaponins with known genins from the Caribbean sponge Erylus formosus.41 Of the five new saponins, scillanostasides A–E, isolated from the bulbs of Scilla scilloides, only A and B have new genins 39 and 40.42 Lanostan-3β-ol 41 is a new genin of a diglucuronoside from the flowers of Punica granatum.43
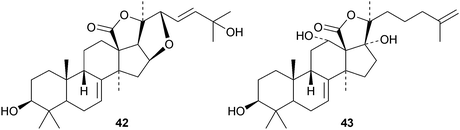
Cucumariosides H5, H6, H7 and H8 are new holostane glycosides from the sea cucumber Eupentacta fraudatrix.44 Cucumarioside H8 has a new genin 42 with an unusual 16,22-epoxide. Patagonicosides B and C, sulphated glycosides from the sea cucumber Psolus patagonicus, display antifungal activity.45 Patagonicoside B has the new genin 43. Two new glycosides with known genins, liouvillosides A4 and A5, have been isolated from the sea cucumber Staurocucumis liouvillei.46
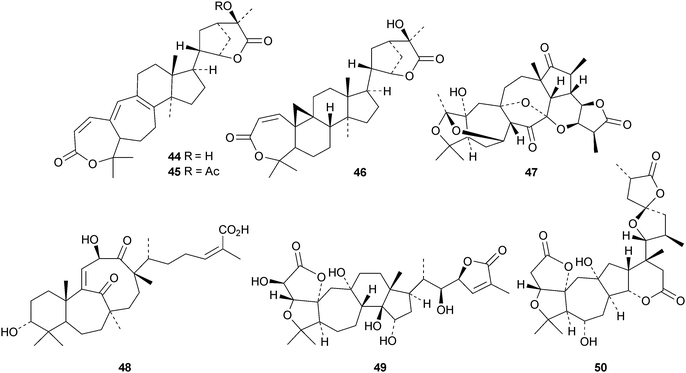
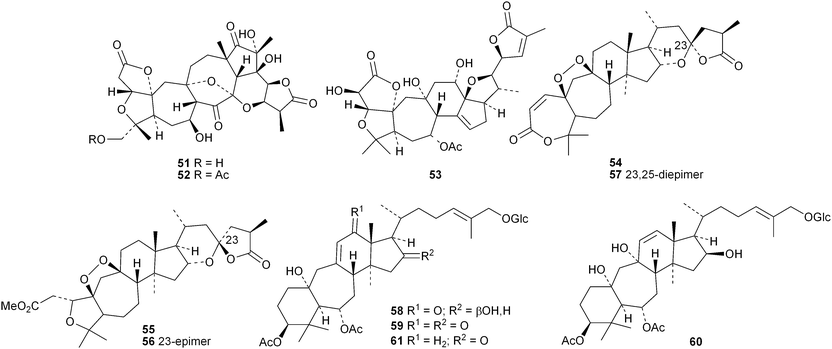
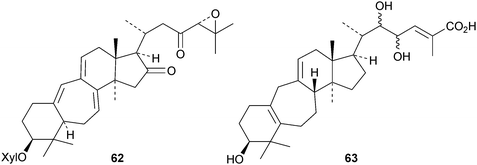
Interesting new compounds from Schisandra species include henrischinins A–C 44–46 from Schisandra henryi with an oxabicyclo[3.2.1]octane moiety in the side chain,47 the bisnor-derivative schinarisanlactone A 47 from Schisandra arisanensis48 and the tricyclic derivative schiglautone A 48 from the stems of Schisandra glaucescens.49 The structure of henrischinin B 45 was confirmed by X-ray analysis. 2β-Hydroxymicrandilactone C 49,50 schintrilactone C 5051 and wilsoniadilactones D–F 51–5352 are new constituents of Schisandra chinensis, Schisandra sphenanthera and Schisandra wilsoniana, respectively. Four new peroxy-lactones, pseudodarolides Q254, T155 and T25653 and 25-epipseudolarolide Q 57,54 have been isolated from Pseudolarix kaempferi. Huangqiyenins G–J 58–61 are new saponins from Astragalus membranaceus.55 The xyloside cimipodocarpaside 62 has been reported from Cimifuga racemosa.56 The myxomycete Tubulifera arachnoidea afforded the new 9,10-secocycloartane tubiferic acid 63.57
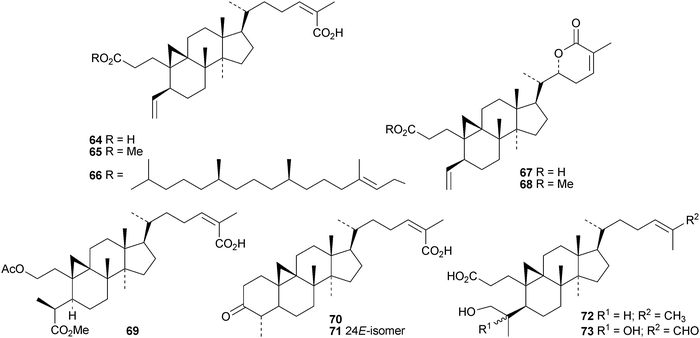
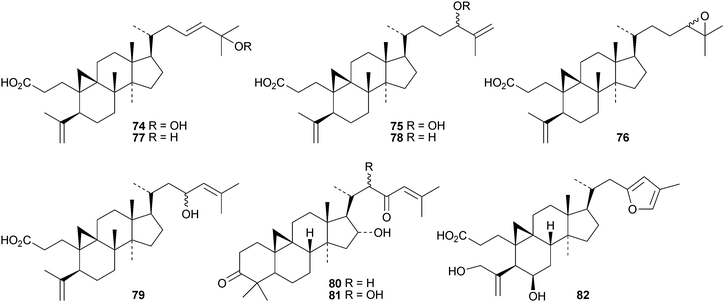
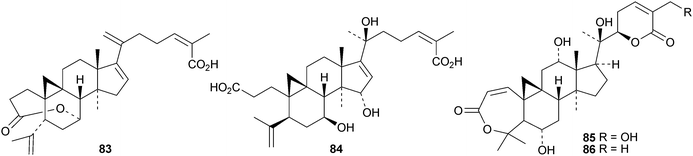
Sinocalycanchinensins A–H 64–71 are 29-norcycloartanes from the leaves of Sinocalycanthus chinensis.58 Sinocalycanchinensins A–E 64–68 are 3,4-seco-derivatives while sinocalycanchinensin F 69 has a 2,3-cleaved ring A. Other 3,4-cleaved cycloartanes include gardenoins I 72 and J 73 from the exudates of Gardenia thailandica59 and coccinetanes B–G 74–79 from Kadsura coccinea.60 Secopisonic acid from Pisonia umbellifera61 and gardenoin H from the apical buds of Gardenia obtusifolia62 are identical with coccinetane E 77. Gardenoins E–G 80–82 are other constituents of Gardenia obtusifolia.62 Angustific acid A 83, from Kadsura angustifolia, has an unusual bridged lactone.63 It is accompanied by angustific acid B 84 and angustifodilactones A 85 and B 86. The compounds are reported to have anti-HIV activity.
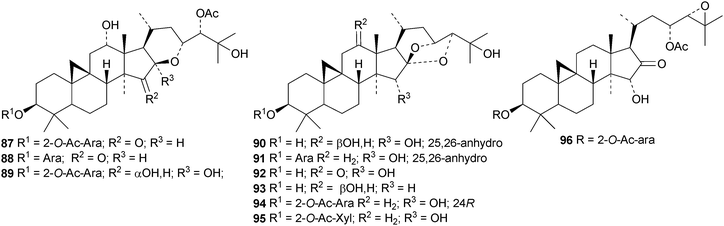
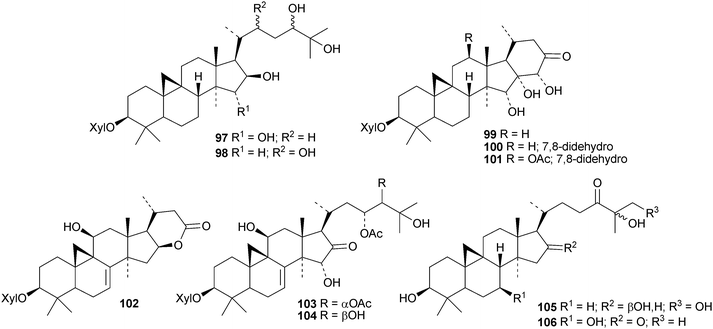
In separate studies ten new cycloartanes and glycosides87–9664 and three new glycosides, two (97 and 98) with new genins,65 have been reported from Cimifuga foetida. Six new glycosides99–104 have been isolated from the rhizome of Cimifuga heracleifolia66 and two, tareciliosides L and M with new genins 105 and 106, from the leaves of Tarenna gracilipes.67 Tareciliosides H–K have known genins.
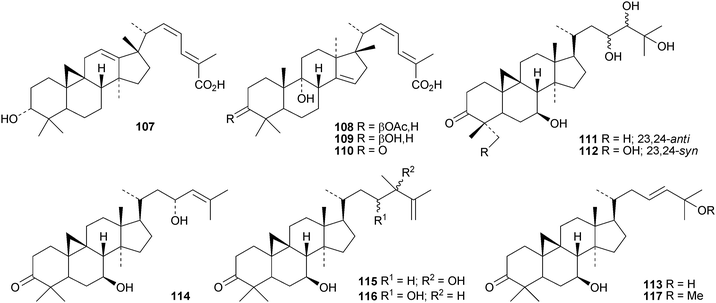
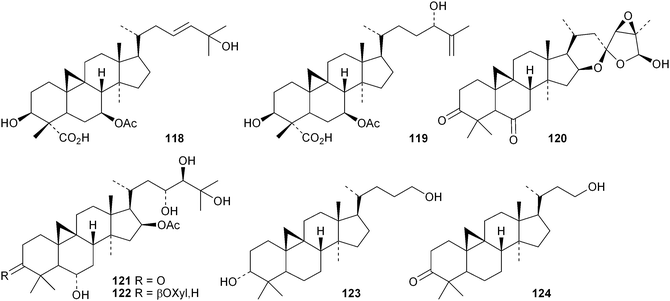
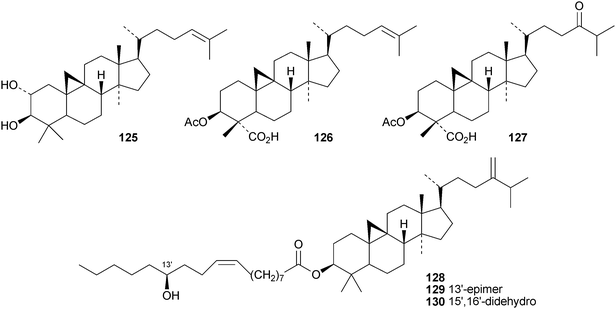
The 18(13→17)-abeocycloartane 107 is a constituent of the bark and leaves of Garcinia benthami, where it occurs along with the 14,17-friedolanostanes 108–110.68 Other new cycloartanes include combretanones A–G 111–117 and combretic acids A 118 and B 119 from Combretum quadlangulare,69 bicusposides D–F 120–122 from Astragalus bicuspis,70 macrostachyosides A 123 and B 124 from Mallotus macrostachyus,71 cycloart-24-ene-2α,3β-diol 125 from the stigma of Zea mays72 and boniatic acids A 126 and B 127 from Radermachera boniana.73 Bonianic acids A 126 and B 127 showed some antitubercular activity. Codonopilates A–C 128–130 are cycloartane esters from Codonopsis pilosula.74
Novel cycloartanesaponins with known genins include askendoside K from Astragalus taschkendicus,75 cicerosides A and B from Astragalus cicer,76 shengmaxinsides A–C from Cimicifuga simplex,77 and unnamed saponins from Astragalus mucidus.78 The biological activities of cycloartanetriterpenoids have been reviewed.79
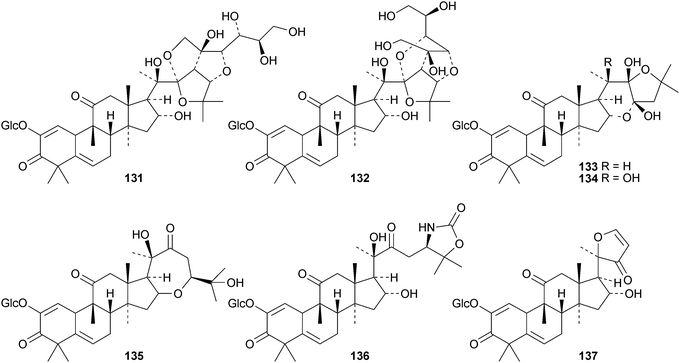
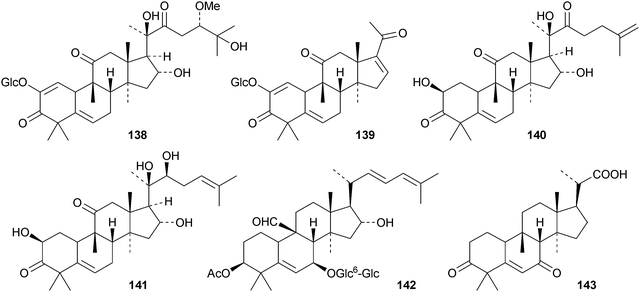
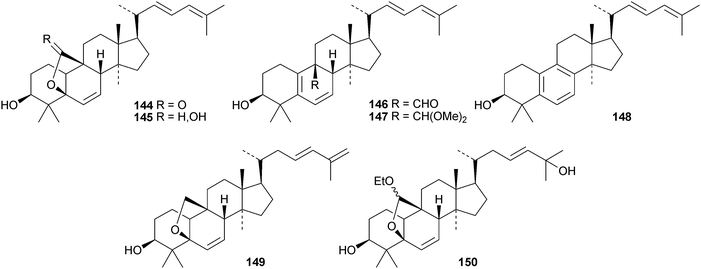
Machilusides A 131 and B 132, from the stem bark of Machilus yaoshansis, are cucurbitane glycosides with an unusual C-glycoside moiety.80 The roots of Machilus yaoshansis afforded seven new glycosides133–139.81 These authors also revised the C-24 configurations of several known compounds, including cucurbitacins S and T and colocyhthins A, B and C, from 24S to 24R. Compounds 140 and 141, from the roots of Wilbrandia ebracteata, are reported to have cytotoxic activity.82 New cucurbitanes from Momordica charantia include the antioxidants taiwacins A 142 and B 143 from the stems and fruit,83144–14884 and 149 and 150.85 Compound 148 is a 19-nor-derivative with an aromatic ring B. The biological activities of compounds from Momordica charantia have been reviewed.86
4 The dammarane group
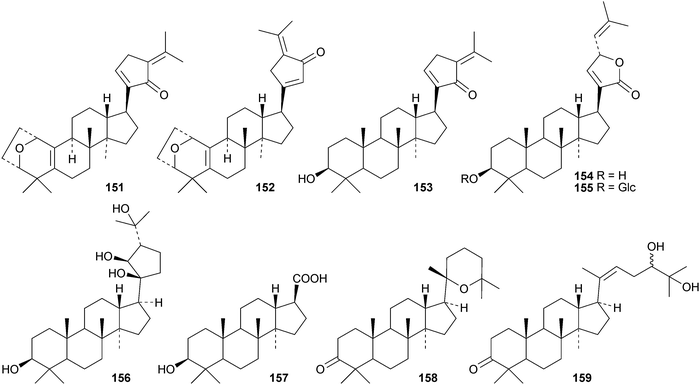
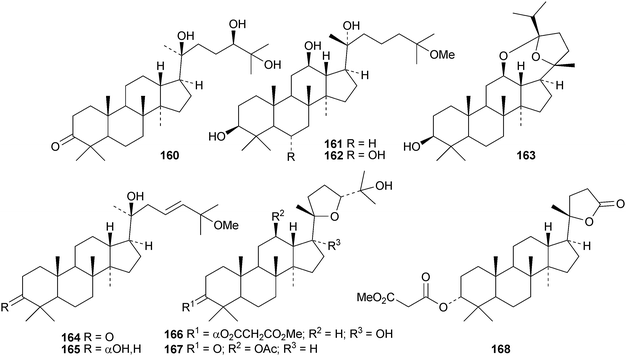
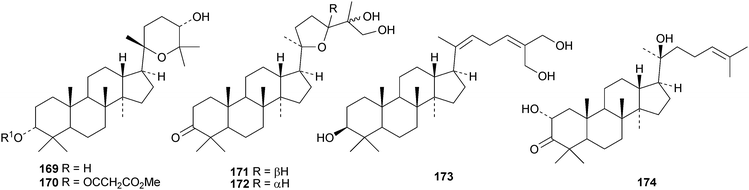
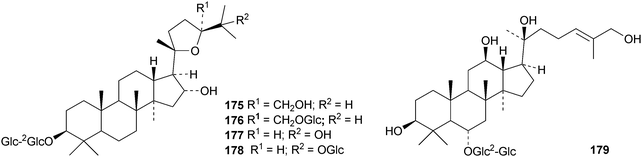
Gypensapogenins A 151 and B 152 are modified dammaranes, with an unusual ring A, from Gynostemma pentaphyllum, where they are found with gypensapogenins C 153, D 154 and the glucoside15587 and the 21,24-cyclo derivative 156 and the nonanordammarane 157.88 The structure of gypensaponin A 151 was confirmed by X-ray analysis. Other new dammaranes include gardaubryones A–C 158–160 from Gardenia aubryi,89161–163 from the berries of Panax ginseng,90164–170 from the floral spikes of Betula platyphylla var. japonica,91 the 24-epimers 171 and 172 from the apical buds of Gardenia collinsae,92 dammara-20(22),24-diene-3β,26,27-triol 173 from the leaves and twigs of Rhus taitensis25 and the α-ketol 174 from the exudate of the leaves of Cerasus yedoensis.93 The structure recently proposed for ailexcelone, from Ailanthus excelsa, is similar to that of gardaubryone B 159 but its spectroscopic data are inconsistent with this structure, The revised structure, 24,25-dihydroxytirucall-7-en-3-one, has been proposed and the structure of the corresponding 3β-hydroxy-derivative should also be revised.89Four new saponins, operculinosides A–D 175–178, have been reported from the aerial parts of Operculina turpethum.94 The structure of operculinoside A175 was confirmed by X-ray analysis. Of the six saponinsginsenosides Re1- Re6 have been reported from the root of Panax ginseng, only ginsenoside Re5179 has a new genin.95 Panajaponol, from the roots of Panax japonicus var. major, is identical to ginsenoside Re5179 but was drawn with the wrong double bond geometry.96 Reviews on the pharmacological activities of the ginsenosides have appeared.97,98
Novel dammaranesaponins with known genins include betalnosides B and C from Betula alnoides,99 centellosides A and B and ginsenosides Mc and Y from Centella asiatica,100ginsenosides Ra4–Ra9101 and 20R-ginsenoside ST2102 from Panax ginseng, gypenosides GC1–GC7 from Gynostemma pentaphyllum,103 notoginsenosides SFt1–SFt4 from Panax notoginseng,104 pseudoginsenosides G1 and G2 from Panax quinquefolium,105 yesanchinosides R1 and R2 from Panax japonicus106 and unnamed saponins from Gynostemma pentaphyllum.107
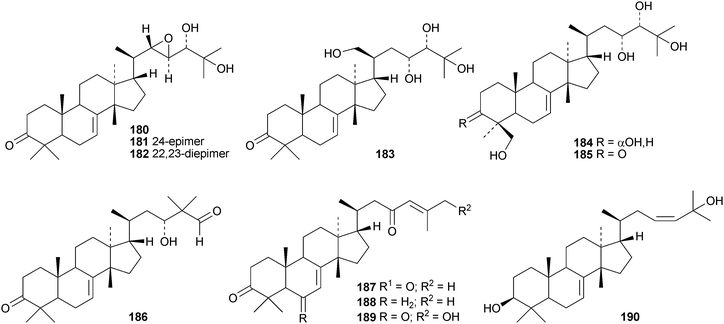
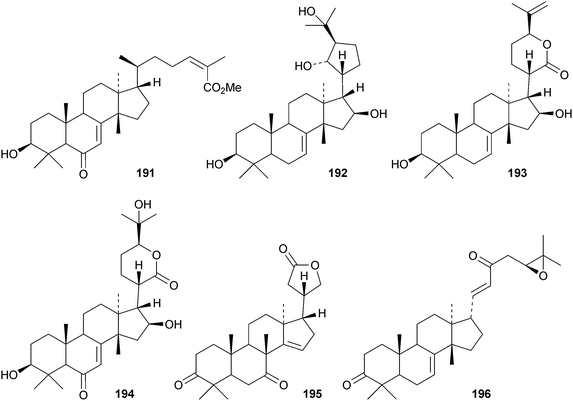
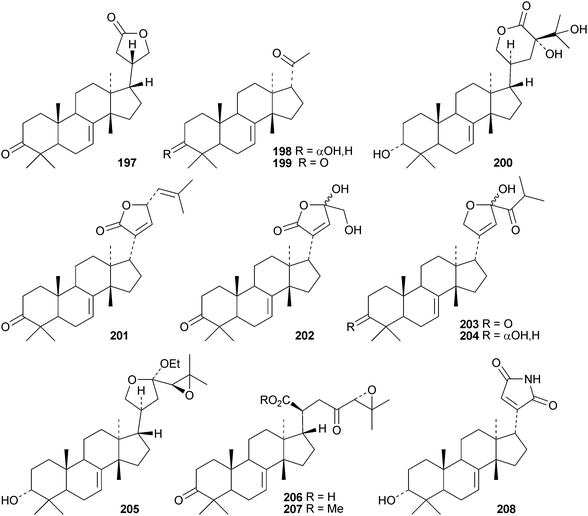
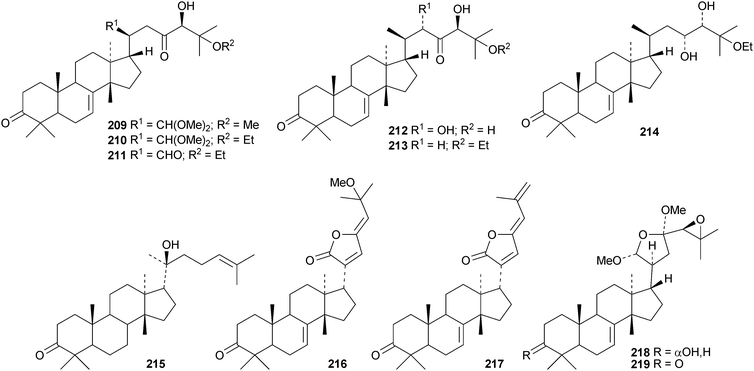
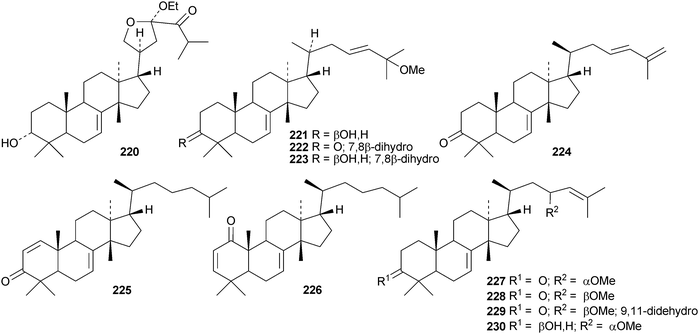
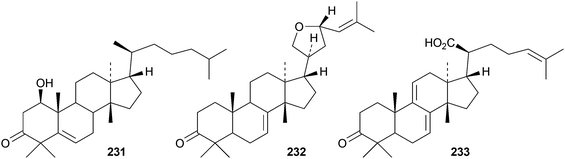
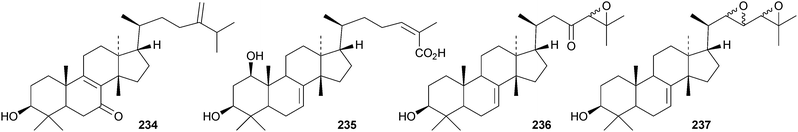
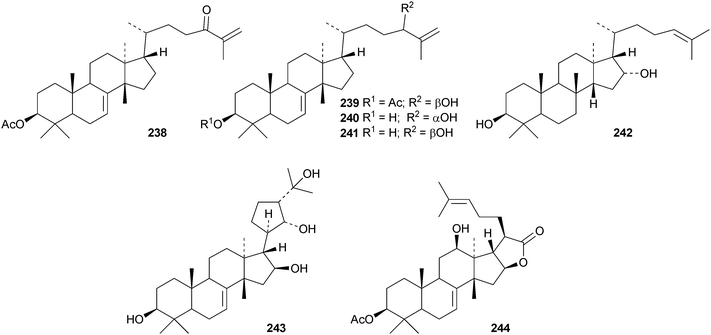
Toona ciliata var. pubescens is the source of the tirucallane derivatives toonapubesins A–G 180–186.108 Toonapubesin G 186 has a rearranged side chain. The tirucallanes 187–192, together with dysoxylumstatins A–C 193–195, have been reported from Dysoxylum lukii.109 Dysoxylumstatin C 195 is an apotirucallane γ-lactone. Several nor-tirucallane derivatives 196–199 have been isolated from Aphanamixis grandifolia.110 Compound 199 was also isolated as dysolenticin G from the twigs and leaves of Dysoxylum lenticellatum, a rich source of interesting tirucallane derivatives including dysolenticin A200, with its rearranged side chain, and dysolenticins B–F 201–205 and H–J 206–208.111 The structures of 200, 202, 203, 205 and 207 were confirmed by X-ray analyses. Other new tirucallane derivatives from Aphanamixis grandifolia include aphagranins A–G 209–215112 and compounds 216–220.113 Several of these compounds look suspiciously like artefacts of the extraction process. Cornus walteri is also a good source of new tirucallane derivatives.114 The constituents of this plant include cornusalterins A–L 221–232. Ailanthusaltenin A, from the stem bark of Ailanthus altissima,115 is the same as cornusalterin D224. Other new tirucallanes include 233 from Euphorbia sapinii,30234 from the resin of Boswellia carterii,116 the dihydroxy acid 235 from Jordanian propolis117 and 236 and 237 from Azadirachta indica.118
Only seven euphane triterpenoids have been reported. They are compounds 238–241 from the bark of Broussonetia papyrifera,119 nepetadiol 242 from Nepeta suavis120 and the 21,24-cycloeuphane 243 and cinamodiol acetate 244 from the bark of Melia azedarach.121
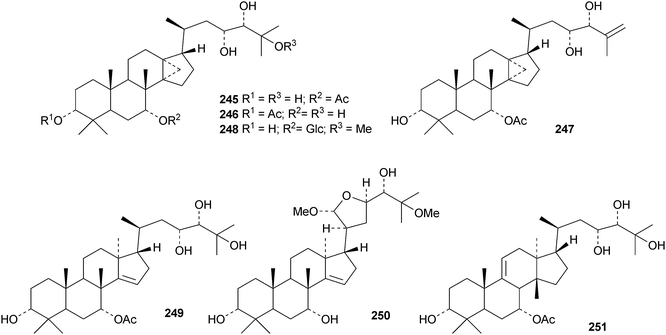
Cumingianols A–C 245–247 are cycloapotirucallane derivatives from Dysoxylum cumingianum.122 Other constituents include cumingianoside R 248, a rare glycoside in this series, the apotirucallane derivatives, cumingianols D 249 and E 250, and the tirucallane, cumingianol F 251.
4.1 Tetranortriterpenoids
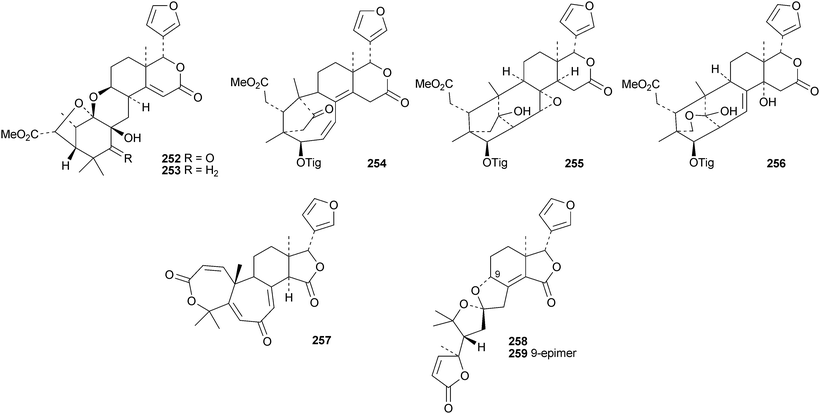
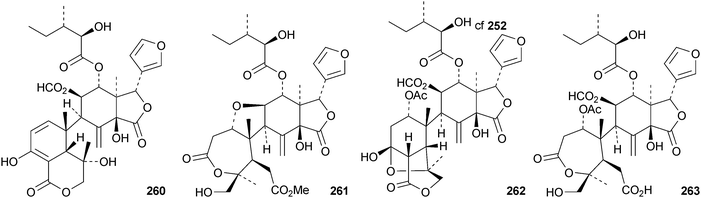
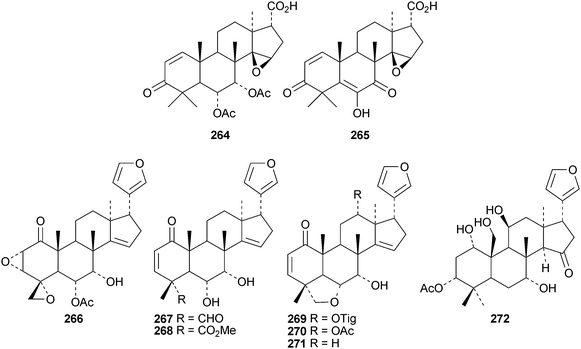
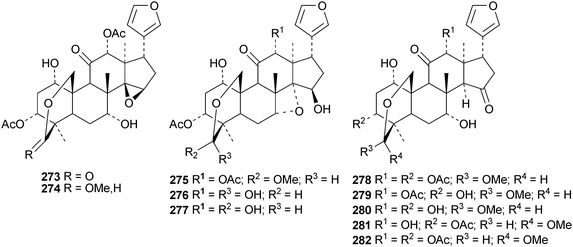
Reviews have appeared on limonoids from the Meliaceae123 and from Trichilia emetica124 and on the synthesis of limonoid natural products.125 Kokosanolides A 252 and C 253 are rearranged limonoids from the seeds and bark of Lansium domesticum cv. Kokossan.126 Other interesting derivatives include chisomicines A 254, B 255 and C 256 from the bark of Chisocheton ceramicus,127 5,6-didehydrodesepoxyhaperforin C2 257 and harrpernoids B 258 and C 259 from the fruit of Harrisonia perforata,128 aphapolynins A 260 and B 261129 and aphanamolides A 262 and B 263130 from Aphanamixis polystachya. The structures of kokosanolide A 252, chisomicines A–C 254–256 and aphapolyrin A 260 were all confirmed by X-ray analyses.The lack of a furan ring is the notable feature of the tris-nor derivatives toonapubesic acids A 264 and B 265 from Toona ciliata var. pubescens.108 The structure of the methyl ester of toonapubesic acid A was confirmed by X-ray analysis. Ceramicines E–I 266–270 constitute a series of 1-oxo derivatives from Chisocheton ceramicus.131 The structure of the previously published ceramicine B 271 has been confirmed by X-ray analysis. Meliarachins A–K 272–282 are further limonoids from the twigs and leaves of Melia azedarach.132
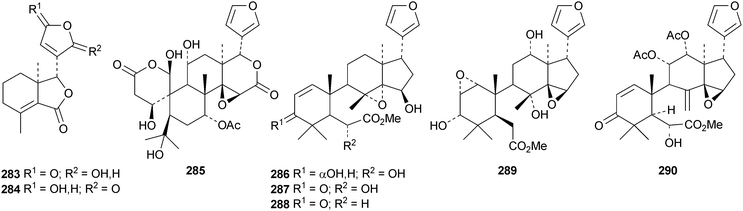
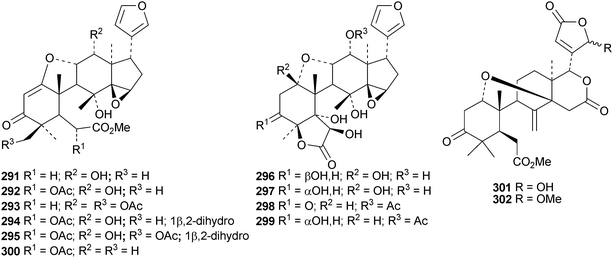
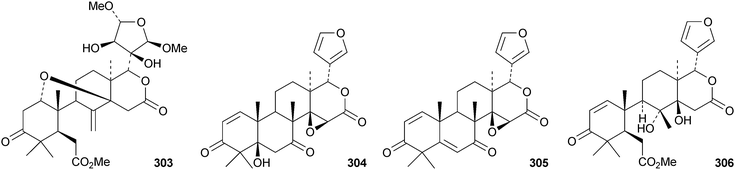
Dasylactones A 283 and B 284 are degraded derivatives from Dictamnus dasycarpus.133 Raputiolide 285 is a ring-A cleaved limonoid from Raputia heptaphylla.134Toona ciliata var. henryi is a rich source of ring-B cleaved derivatives, affording toonacilianins A–L 286–297.135 Toonacilianins K 296 and L 297 are 29-nor derivatives. Two further 29-nor derivatives, toonaciliatins N 298 and O 299 have been reported from Toona ciliata var. yunnanensis, where they occur along with toonaciliatin P 300.136 Three methyl angolensate derivatives 301–303 have been found in the root bark of Entandrophragma angolense, where they occur with the gedunin derivatives 304 and 305.137 Compound 301 is the same as moluccensin O which was published in 2010. Thaimoluccensin A 306 is an andirobin derivative from the seeds of Xylocarpus moluccensis.138 Although its structure was confirmed by X-ray analysis the wrong relative configuration was published in the original paper.
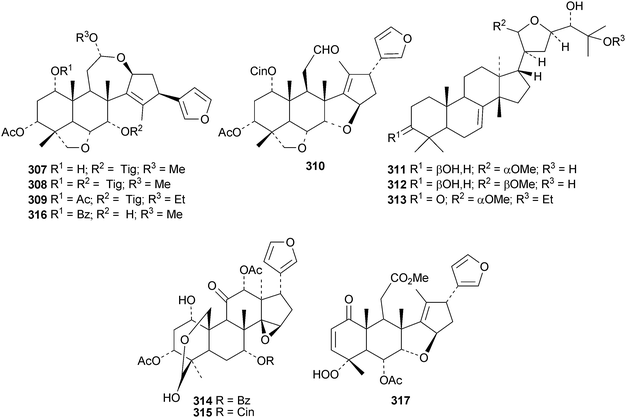
Four new ring C cleaved limonoids 307–310 have been isolated from the fruit of Melia toosendan, together with the tirucallane derivatives meliasenins S 311 and T 312.139 Meliasenin T 312 was also obtained from Melia azedarach seeds where it occurs with the tirucallane 313, the toosendanin esters314 and 315 and the nimbolinin C derivative 316.140 The ring-C cleaved hydroperoxide317 has been isolated from Azadirachta indica.118
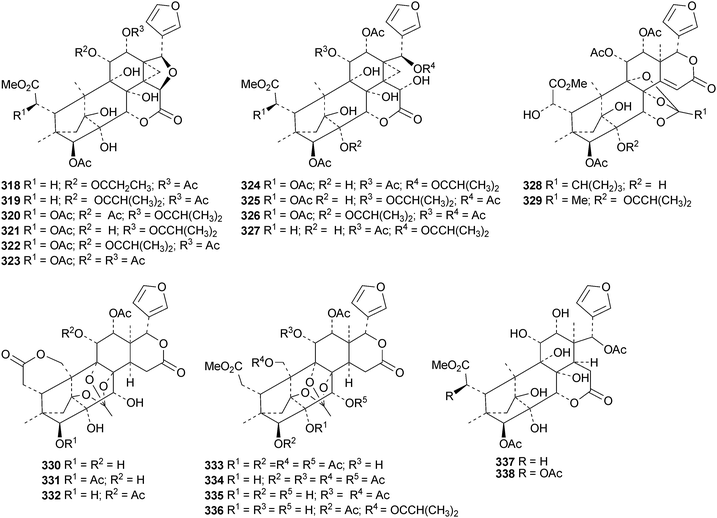
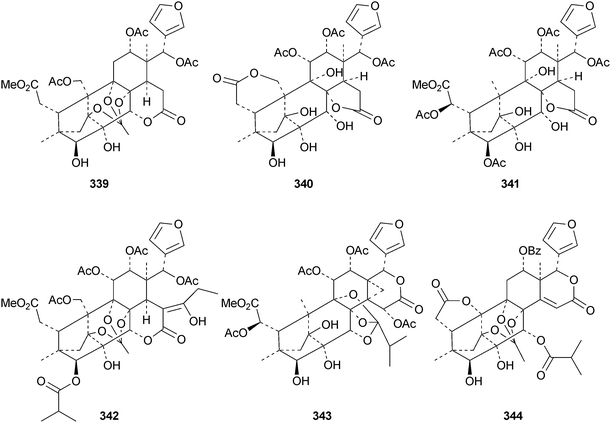
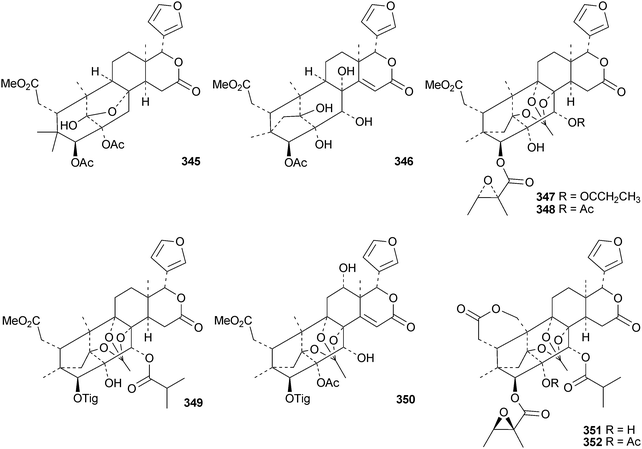
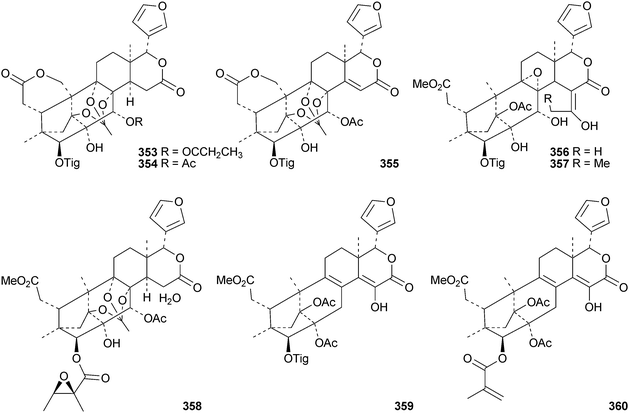
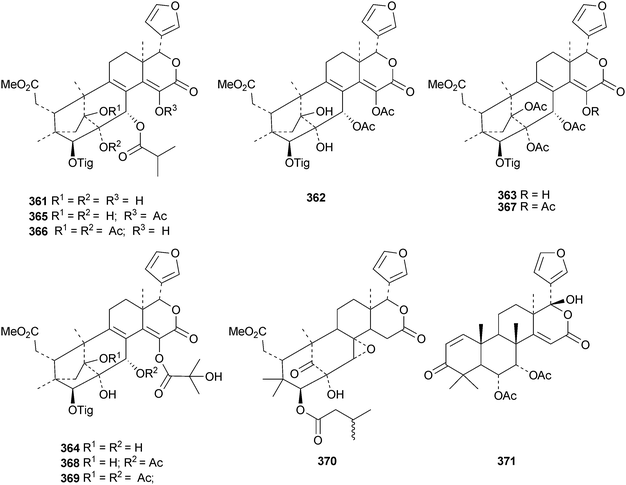
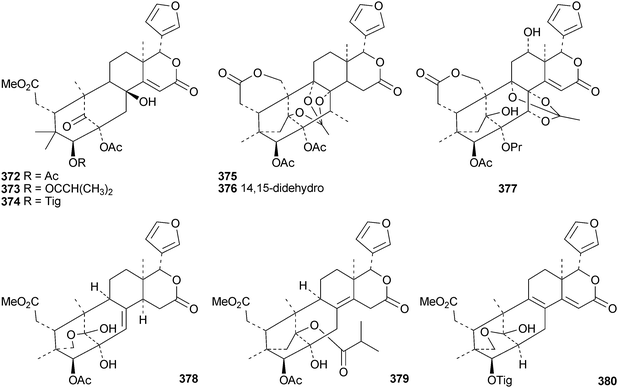
The flow of new mexicanolide and phragmalin derivatives continues unabated. Chukrasia tabularis var. velutina is a particularly rich source. The new derivatives reported from this source include velutabularins A–J 318–327,141 tabulalides F–N 328–336,142 tabulalins A–E 337–341,143 chukvelutilide H 342 and tabularisin R 343,144 tabulvelutins A 344 and B 345145 and tabulalin F 346.146 Many of these compounds are trivial variants of known systems. Velutabularins A–J 318–327 are cyclopropyl derivatives with a modified ring D and tabulvelutin A 344 is a 19-nor derivative. A similar range of phragmalin derivatives, swietenitins N–X 347–357, has been isolated from the twigs of Swietenia macrophylla.147 The structure of swietenitin N 347 was confirmed by X-ray analysis. The stereochemistry of the known compound 14,15-dihydroepoxyfebrinin B 358 was also established during this study. The leaves of Trichilia connaroides produced trichagmalins A–F 359–364 and several acetyl derivatives 365–369, together with trichanolide370.148 The gedunin andirolide A 371, the mexicanolides andirolides B–D 372–374 and the phragmalins andirolides E–G 375–377 have been reported from the flowers of Carapa guianensis.149 The structure of andirolide E 375 was confirmed by X-ray analysis. Other phragmalin derivatives include thaimoluccensins B 378 and C 379 from the seeds of Thai Xylocarpus moluccensis138 and godvarin K 380 from the Godvari mangrove Xylocarpus moluccensis.150
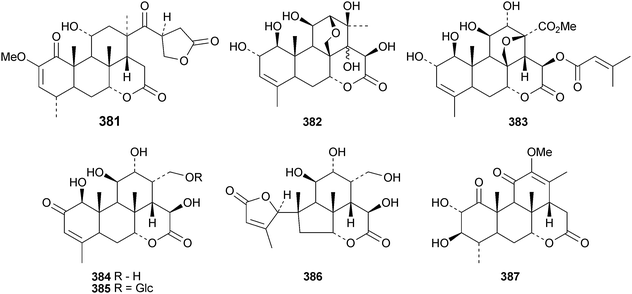
New quassinoids are few in number. They include 2′-isopicrasin A 381 from the stems of Picrasma quassinoides,151 bruceines K 382 and L 383 from the ripe fruit of Brucea javanica,152 yadanziolides T–V 384–386 from the stems of Brucea mollis153 and nothospondin 387 from Nothospondias staudtii.154
5 The lupane group
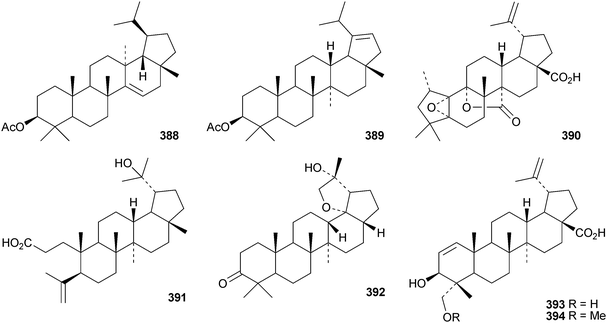
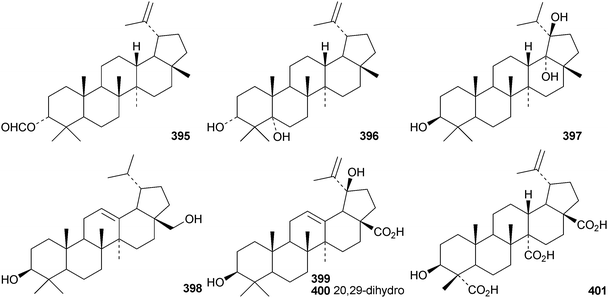
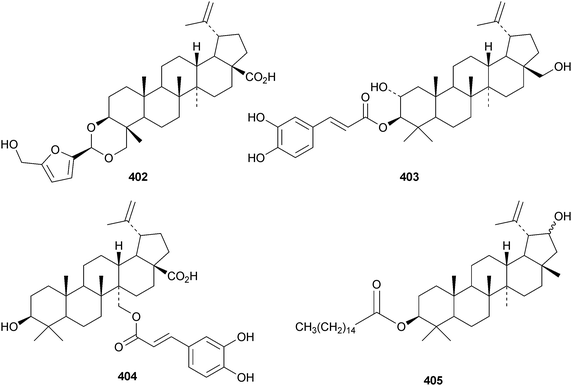
The pharmacological activities of lupeol155 and lupanesaponins156 have been reviewed. Lactucenyl acetate 388, from Lactuca indica, has a migrated lupane structure which is identical to the structure originally assigned to tarolupenyl acetate.157 The structure of tarolupenyl acetate has been revised to lup-19(21)-en-3β-yl acetate 389. Breynceanothanolic acid390 is a 25-nor-ceanothic acid derivative from roots of Breynia fruticosa.158 The ring A-secolupane dysoxyhainic acid H 391 is from Dysoxylum hainanense.159Liquidambar formosana is the source of liquidambarone 392 which is 18α,29-epoxy-20R-hydroxy-28-norlupan-3-one.160 Sorbicins A 393 and B 394 are lupane derivatives from Sorbus cashmiriana.161 Olibanum, the gum resin of Boswellia carterii, is the source of olibanumols F 395 and G 396.162 Other simple lupane derivatives include lupane-3β,18α,19β-triol 397 from Garcinia tetralata,163 lup-12-ene-3β,28-diol 398 from roots of Diospyros virginiana,164 the 3β,19β-dihydroxy derivatives 399 and 400 from Paragonia pyrimidata,165 and the 23,27,28-trioic acid 401 from Heteropanax fragrans.166 Pulsatilla triterpenic acid A 402, from Pulsatilla chinensis, is an acetal of 5-hydroxymethylfurfural and 3β,23-dihydroxylup-20(29)-en-28-oic acid.167 The caffeate esters403168 and 404169 are from Alnus firma and Alangium salviifolium, respectively, while the palmitate ester405 is found in leaves of Rauvolfia vomitoria.170 The 21-configuration of 405 has not been established. Seven lupanesaponins with known genins have been isolated from Stryphnodendron fissuratum.1716 The oleanane group
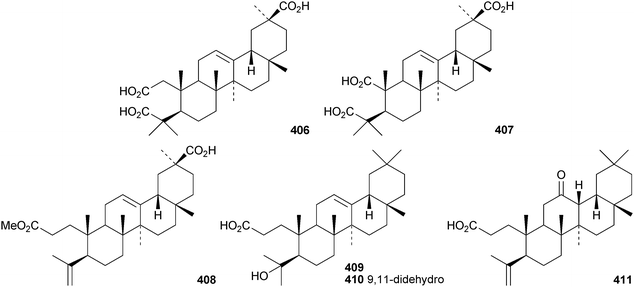
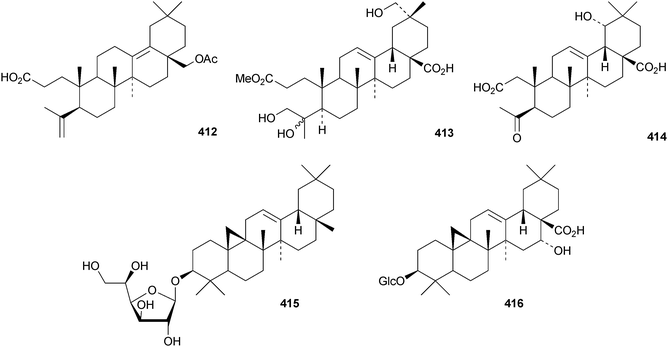
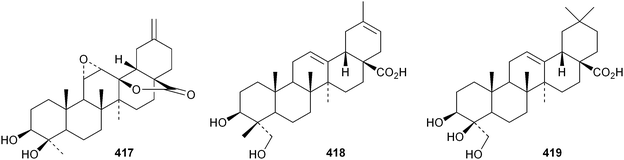
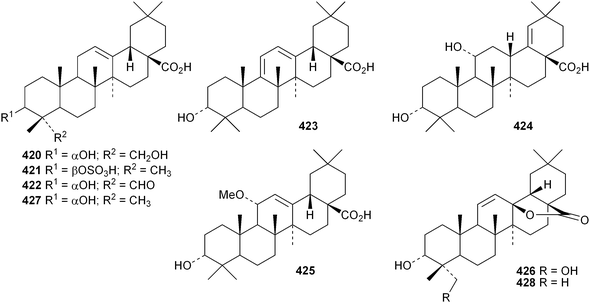
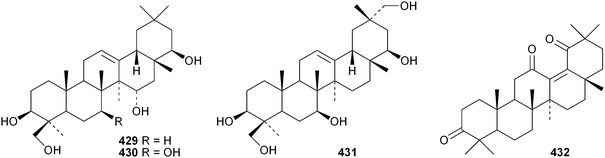
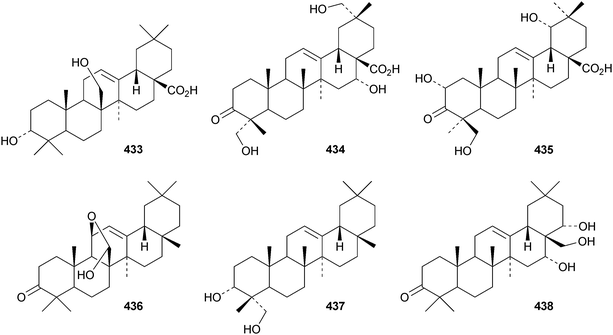
Several ring-A seco-oleananetriterpenoids have been isolated, including the 2,3-seco-oleanenetrioic acid 406 from Dillenia philippinensis,172 dysoxyhainic acid F 407, G 408, I 409 and J 410 from Dysoxylum hainanense,159 the 12-ketone 411 and 13(18)-ene 412 from Betula pendula,173 the 3-methyl ester 413 from Kalopanax pictus174 and ivorengenin B 414 from Terminalia ivorensis.175 The unusual 9,25-cycloolean-12-en-3β-yl β-D-glucofuranoside 415 has been reported to be a constituent of Celestris australis176 and the same group has identified 9,25-cyclo-3β-(β-D-glucopyranosyloxy)-16α-hydroxyolean-12-en-28-oic acid 416 in Symplocos paniculata.177 The 24,30-dinoroleanane417, 30-noroleanane418 and 24-noroleanane419 derivatives are present in the roots of Paeonia rockii ssp. rockii.178 A review covering the structures and pharmacological activity of noroleanane triterpenoids has been published.179 The antitumour activities of oleananetriterpenoids have been surveyed.180Fatsicarpains A–G 420–426 are oleanane derivatives from leaves and twigs of Fatsia polycarpa.181 The structures of fatsicarpain A 420 and the co-occurring known oleananes 427 and 428 were confirmed by X-ray analyses. 15α-Hydroxysoyasapogenol B 429, 7β,15α-hydroxysoyasapogenol B 430 and 7β,29-dihydroxysoyasapogenol 431 are metabolites of the endophytic fungus Pestalotiopsis clavispora, isolated from Bruguiera sexangula.182 The structure of 15α-hydroxysoyasapogenol B 429 was confirmed by X-ray analysis. The structure of olean-13(18)-ene-3,12,19-trione432, from Sedum linare, was also established by X-ray analysis.183
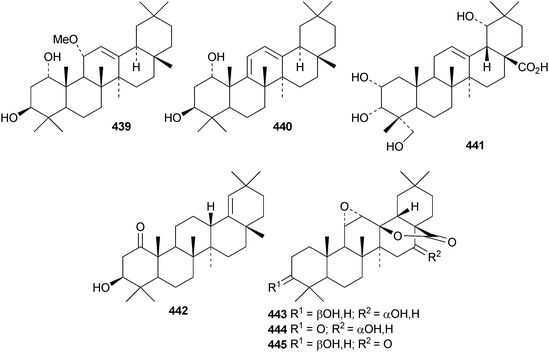
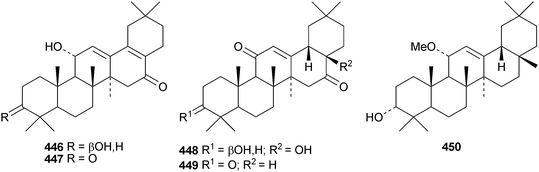
Other new simple oleanane derivatives include ambradiolic acid A 433 from Liquidambar formosana,160 16α,23,29-trihydroxy-3-oxoolean-12-en-28-oic acid 434 from Kalopanax pictus,174 ivorengenin A 435 from Terminalia ivorensis,175 salacetal 436 from Salacia longipes var. camerunensis,184 olean-12-ene-3α,23-diol 437 from Salvia miltiorrhiza,185 camelliagenone 438 from Barringtonia asiatica,186 the 1,3-diols 439 and 440 from Viburnum chingii,187 2α,3α,19α,23-tetrahydroxyolean-12-en-28-oic acid 441 from Rosa laevigata,188 3β-hydroxyolean-18-en-1-one 442 from Juglans chinensis,189443–449 from Nannoglottis carpesioides190 and olibanumol E 450 from olibanum, the gum resin of Boswellia carterii.162
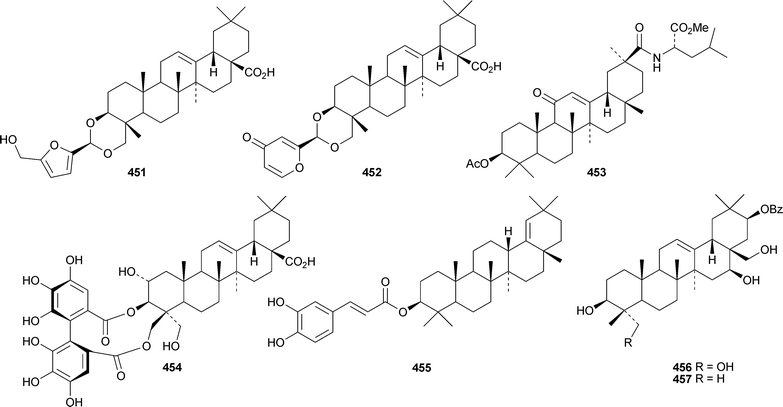
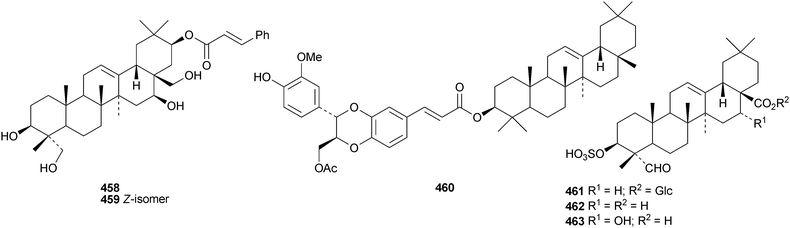
Pulsatilla triterpenic acids B 451 and C 452, from Pulsatilla chinensis, are hederagenin acetal derivatives.167 The first glycyrrhetic acid amino acid conjugate, dendrophen 453, has been isolated from Dendronephthya hemprichi.191 The aglycone 454 of the known castanopsinin Ea has been found in leaves of Castanopsis fissa.192 Other new oleanane ester derivatives include the caffeoyl ester of germanicol455 from Barringtonia asiatica,186esters456–459 from Glochidion assamicum,193 uragogin 460 from Crossopetalum uragoga,194 and the sulfateesters461–463 from Gypsolphila pacifica.195
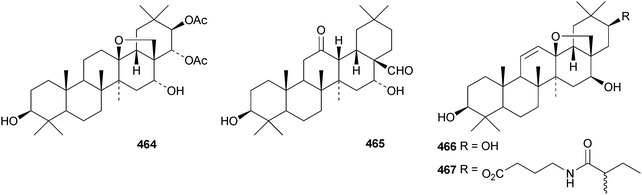
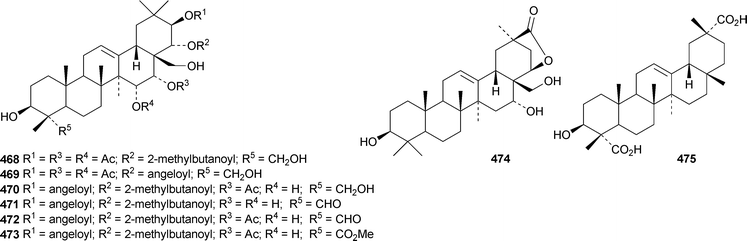
Clethroidosides A–G are oleananesaponins from Lysimachia clethroides.196 Clethroidosides F and G have the new genins 464 and 465, respectively; the others have known genins. Heterogenoside F, from Lysimachia heterogenea, is identical to clethroidoside F and it is found with heterogenoside E that has a known genin.197 The genins of glaucasides A and B, from Atriplex glauca var. ifiniensis, are the new compounds 466 and 467 whereas glaucaside C has the known genin saikogenin G. Camellia sinensis is a rich source of saponins including rogchaponins R1–R10.198 Rogchaponins R1, R2 and R4–R7 have the new genins 468–473, respectively. Myrseguinoside D, from Myrsine seguinii, has the new genin 474.199 It is accompanied by myrseguinoside E which is the same as the known ardisicrenoside J. Dianthosaponins A–F are found in Dianthus japonicus.200 Dianthosaponins E and F have the new genins 475 and 476, respectively. Bridgesides A1, C1, C2, D1, D2, E1 and E2, from Echinopsis macrogona, include the new genins 477 and 478.201 Other oleananesaponins with new genins include 3β,12β,30-trihydroxyoleanan-28,13β-olide 479 from Patrinia scabiosaefolia,202 the esters480 and 481 from Maesa lanceolata,203 oleana-11,13(18)-diene-3β,16α,21β,28-tetrol 482 and the corresponding 21 ketone483 from Bupleurum falcataum and Bupleurum rotundifolium,204 and coryternic acid 484 from Corydalis ternate.205
New oleananesaponins with known genins that have been assigned trivial names are listed in Table 1.
| Trivial name | Plant species | Reference |
|---|---|---|
| Androsacin | Androsace integra | 206 |
| Apodytines A–F | Apodytes dimidiata | 207 |
| Ardisicrenosides I, J, M | Ardisia crenata | 208 |
| Ardisicrenoside N | Ardisia crenata | 209 |
| Azafrines 1, 2 | Crocus sativus | 210 |
| Bifinosides A–C | Panax bipinnatifidus | 211 |
| Blighosides A–C | Blighia sapida | 212 |
| Caraganosides C, D | Caragana microphylla | 213 |
| Caryophyllacosides A, B | Gypsophila paniculata | 214 |
| Catunarosides A–D | Catunaregam spinosa | 215 |
| Catunarosides E–H | Catunaregam spinosa | 216 |
| Celosins A, B | Celosia argentea | 217 |
| Celosins E, G | Celosia argentea | 218 |
| Dexyloprimulanin | Labisia pumila | 219 |
| Dialiumoside | Dialium excelsum | 220 |
| Dipsacus saponins J, K | Dipsacus asper | 221 |
| Elatoside L | Aralia elata | 222 |
| Esculentoside T | Phytolacca acinosa | 223 |
| Gordonosides I–P | Gordonia chrysandra | 224 |
| Halimodendrin I | Halimodendron halodendron | 225 |
| Libericosides A1, A2, B1, B2, C2 | Atroxima liberica | 226 |
| Lonimacranthoide I | Lonicera macranthoides | 227 |
| Mandshunosides A, B | Clematis mandshurica | 228 |
| Micranthosides A–C | Polygala micrantha | 229 |
| Mollusides A, B | Albizia mollis | 230 |
| Onjisaponin Wg | Polygala tenuifolia | 231 |
| Parkiosides A–C | Butyrospermum parkii | 232 |
| Platycoside O | Platycodon grandiflorum | 233 |
| Pseudoginsenoside RT1 butyl ester | Panax japonicus var. major | 96 |
| Puberosides C–E | Glochidion puberum | 234 |
| Rheedeiosides A–D | Entada rheedei | 235 |
| Scoposides F, G | Cephalaria spp. | 236 |
| Umbellatosides A, B | Hydrocotyle umbellata | 237 |
The sources of new oleanane saponins with known genins that have not been assigned trivial names are listed in Table 2.
| Plant species | Reference |
|---|---|
| Albizia inundata | 238 |
| Anemone rivularis var. flore-minore | 239 |
| Anemone taipaiensis | 240 |
| Aralia elata | 241 |
| Arenaria montana | 242 |
| Bellis perennis | 243 |
| Catunaregam spinosa | 244 |
| Cylicodiscus gabunensis | 245 |
| Dianthus superbus | 246 |
| Erthrophleum fordii | 247 |
| Ganoderma applanatum | 248 |
| Gymnocladus chinensis | 249 |
| Gypsophila perfoliata | 250 |
| Juglans sinensiss | 189 |
| Kalopanax pictus | 174 |
| Lathyrus rattan | 251 |
| Medicago polymorpha | 252 |
| Microsechium helleri | 253 |
| Nephelium lappaceum | 254,255 |
| Panacis majoris | 256 |
| Phytolacca americana | 257 |
| Salsola imbricata | 258 |
| Sanguisorba tenuifolia var. alba | 259 |
| Symplocos caudata | 260 |
| Symplocos lancifolia | 261 |
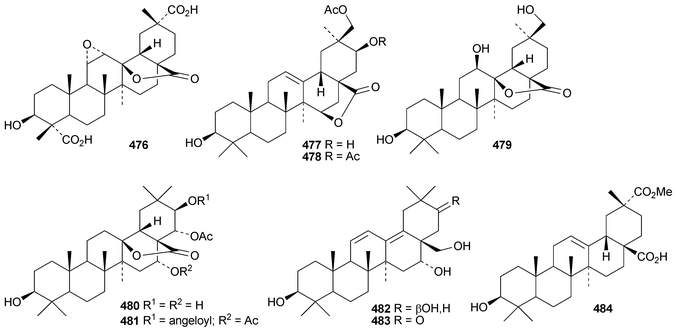
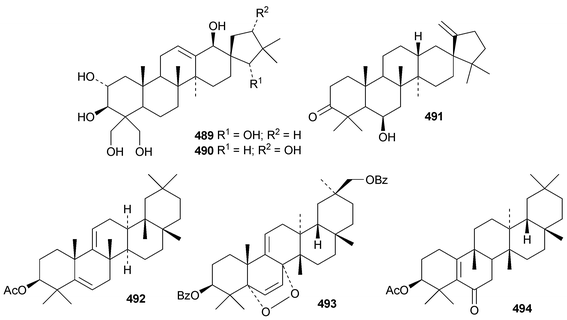
Pachanosides C1, E1, F1 and G1 are pachanane saponins from Echinops macrogona with the genins 485–488, respectively.201 The structure of pachanol C 485 has been revised.
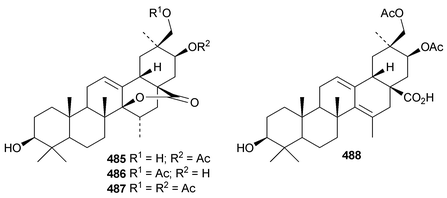
The rearranged oleanane derivatives phlomishexaols C 489 and D 490 have been found in the roots of Phlomis umbrosa.262,263 The biosynthetic origin of the spirotriterpenoid cleistanone 491, from Cleistanthus indochinensis, is not clear from its structure.263 The rearranged oleanane derivative 492 has been claimed from Rhododendron campanulatum.264 The stereochemistry of the methyl group at C-18 of 492 is unusual. The multiflorane endoperoxide dibenzoate 493 is a constituent of processed seeds of Trichosanthes kirilowii.265 3β-Acetoxyglutin-5(10)-en-6-one 494 has been found in roots of Scorzonera austriaca.266
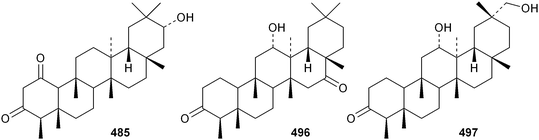
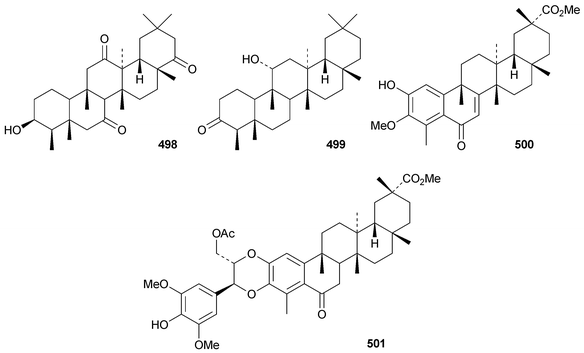
New friedelanetriterpenoids include 21α-hydroxyfriedelane-1,3-dione 495 from Salacia verrucosa,267 12α-hydroxyfriedelane-3,16-dione 496268 and 12α,29-dihydroxyfriedelan-3-one 497269 from Maytenus gonoclada, 3β-hydroxyfriedelane-7,12,22-trione 498 from Drypetes laciniata270 and 11α-friedelan-3-one 499 from Myrica rubra.271 The norfriedelane derivative 3-O-methyl-6-oxopristimerol 500 is a constituent of Maytenus chubensis.272 Blepharodin 501, from Maytenus magellanica, is an adduct with a phenylpropanoid derivative.194
7 The ursane group
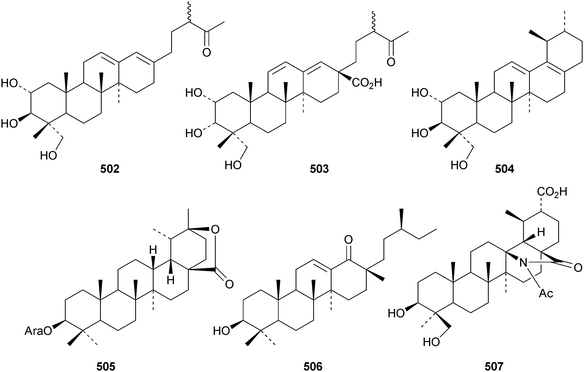
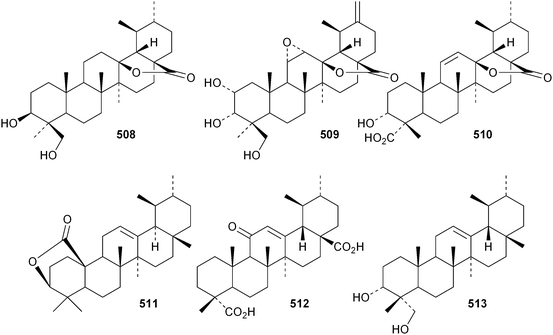
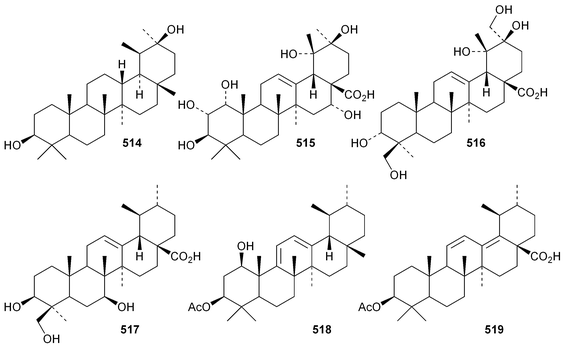
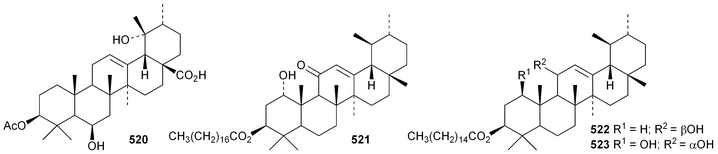
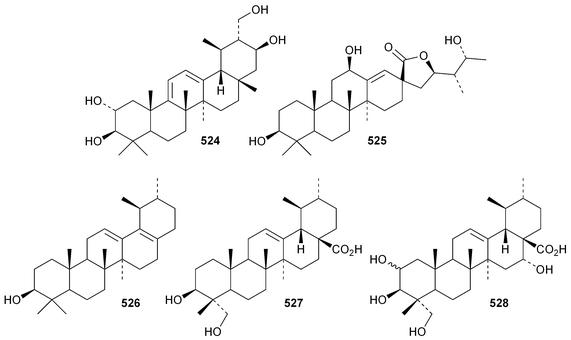
The 18,19-secoursane derivatives 502 and 503 have been isolated from Rosa laevigata together with 28-norursa-12,17-diene-2α,3β,23-triol 504 and the arabinoside505 whose genin has an unusual 19α-stereochemistry.188 The related 18,19-secoursane derivative 506 has been reported from leaves of Diospyros kaki.273 Atriplicaide A 507 is an unusual N-acetyl lactam from Zygophyllum eurypterum where it is found with atriplicaide B 508 which is 3β,24dihydroxyursan-28,13-olide.274 Related 28,13-olides 509 and 510 have been isolated from Isodon coetsa275 and Schefflera heptaphylla,276 respectively. Proceraursenolide 511, from the roots of Calatropis procera, is claimed to be 18αH-urs-12-en-25,3β-olide.277 Other new simple ursane derivatives include cordinoic acid 512 from Cordia latifolia,278 urs-12-ene-3α,23-diol 513 from Salvia miltiorrhiza,185 18αH-ursene-3β,20β-diol 514 from Boswellia carterii,116 1α,2α,3β,16α,19α,20β-hexahydroxyurs-12-en-28-oic acid 515 from Pedicularis kansuensis,279 glutinolic acid 516 from Rehmannia glutinosa,280 and 3β,7β,24-trihydroxyurs-12-en-28-oic acid 517 from Saurauja roxburghii.281 New ursane ester derivatives include conrauidienol 518 from Ficus conraui,282 3β-acetoxyursa-11,13(18)-dien-28-oic acid 519 from Eucalyptus camaldulensis,2833-O-acetyluncaric acid520 from Radermachera boniana,73 sambucilate 521 from Sambucus adnata284 and the palmitate esters522 and 523 from Viburnum betulifolium.285Clethroidoside H, from Lysimchia clethroides, is an ursane saponin with the new genin ursa-9(11),12-diene-2α,3β,21β,30-tetrol 524.196 The 18,19-secoursane derivative 525 is the genin of dunnianolactones A–C from Ilex dunniana.286 A saponin given the duplicate name ilexsaponin C, from Ilex pubescens, has the new genin 28-norursa-12,17-dien-2β-ol 526.287 Other unnamed ursanesaponins with the new genins include 3β,23-dihydroxyurs-12-en-28-oic acid 527 from Jugalans sinensis,189 and 2,3β,16α,23-tetrahydroxyurs-12-en-28-oic acid 528 from Lathyrus aphaca.251
Ursane saponins with known genins include asiaticoside G from Centella asiatica,288 clethric acid 28-O-β-D-glucopyransyl ester and mussaendoside T from Anthocephalus chinensis,289 ilekudinchosides A–D290 and W291 from Ilex kudincha, symplocosins A and B from Symplocos cochinchinensis var. philippensis,292 zygophylloside S from Zygophyllum coccineum293 and unnamed saponins from Ilex chamaedryfolia,294Juglans sinensis,189Sanguisorba tenuifolia var. alba259 and Symplocos lancifolia.261
19β-Hydroxy-3,4-seco-4(23),20(30)-taraxastadien-3-oic acid 529 has been isolated from buds of Betula pendula.173 The tanninester530 of 2α,3β,23,24-tetrahydroxytaraxastan-28,20β-olide is a constituent of leaves of Castanopsis fissa.192 The taraxastane hemiacetal 531 has been found in Geum japonicum.295 Celosin F 532 appears to be a taraxastane xyloside from Celosia argentea.218
8 The hopane group
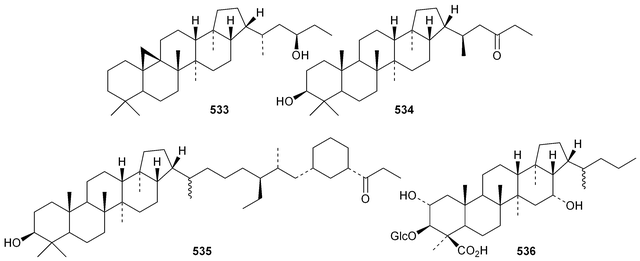
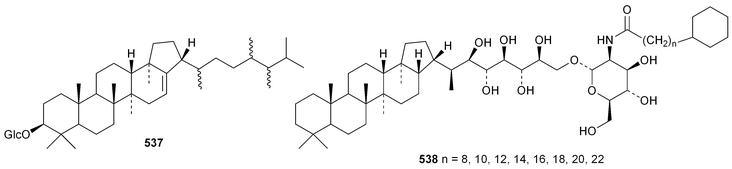
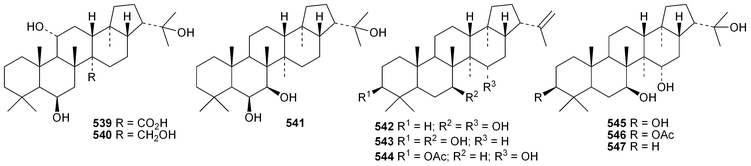
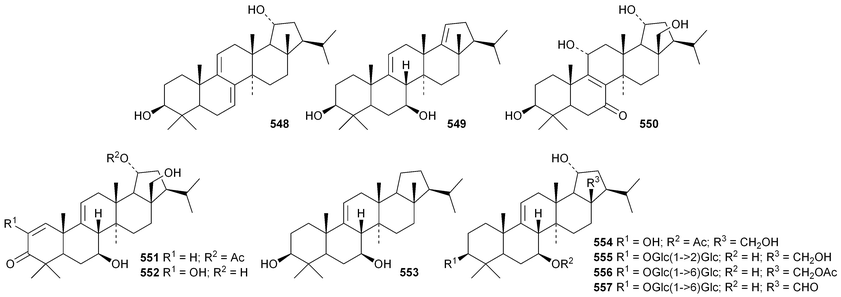
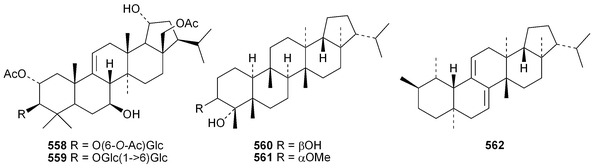
The current knowledge of squalene-hopene cyclases has been reviewed.296 The unusual 9,25-cyclo-29-propylhopan-31-ol 533 and 3β-hydroxy-29-propylhopan-31-one 534 have been identified in Celestris australis.176 The same group claim that the cyclohexylhopane derivative 535 is also found in Celestris australis297 and that the 29-ethylhopane derivative 536 and 32,33,34-trimethylbacteriohopan-3β-yl β-D-glucopyranoside 537 are constituents of Symplocos paniculata.177 Several N-acylated bacteriohopanehexol mannosamine derivatives 538 have been identified in the thermophilic bacterium Alicyclobacillus acidoterrestris.298 The simple hopane derivatives 539–541299 and 542–54738 are metabolites of the entomopathogenic fungi Conoideocrella tenuis and Hypocrella sp. BCC 14524, respectively.Twelve arborinane triterpenoids have been isolated from Rubia yunnanensis including rubiyunnanols A–C 548–550, rubiarbonone E 19-acetate 551, 2-hydroxyrubiarbonone E 552, 19,28-didehydroxyrubiarbonol A 553, rubiarbonol A 7-acetate 554, the rubiarbonol A glycoside 555, rubiarboside G 28-acetate 556, rubiarboside G 28-aldehyde 557, 2α-acetoxyrubiarboside 28-acetate 558 and the rubianol E glycoside559.300Adiantum capillus-veneris is the source of filicane-3β,4α-diol 560 and the corresponding 3α-methyl ether 561.301 Canarene 562 is an unusual rearranged filicane derivative from Canarium schweinfurthii.302 The structure of canarene 562 was confirmed by X-ray analysis and a biosynthetic scheme for its formation has been proposed.
9 Miscellaneous compounds
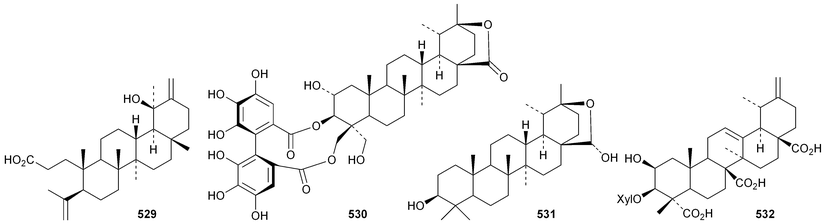
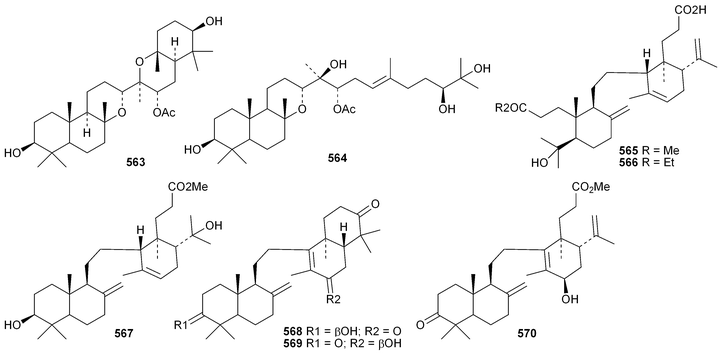
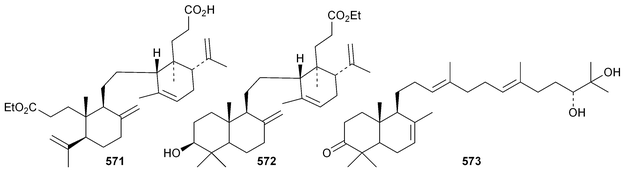
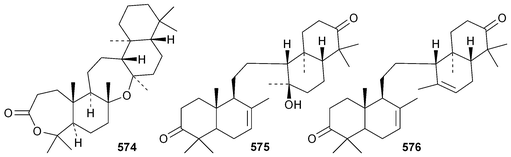
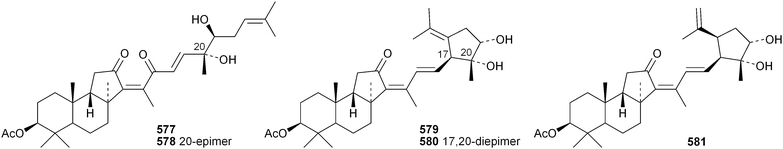
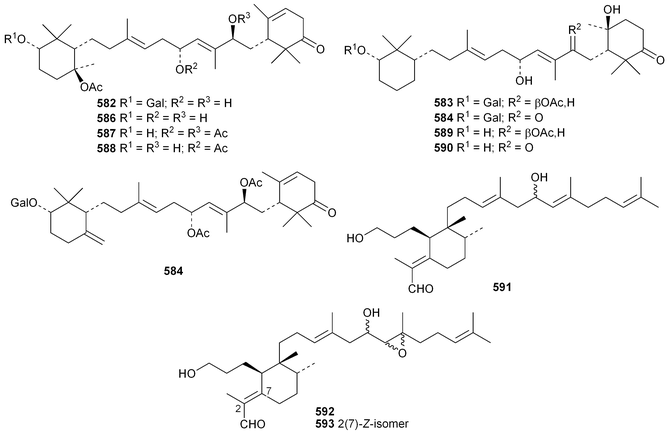
Phyteumosides A and B, from Phyteuma orbiculare, have as aglycones the partially cyclised onocerane triterpenoid563 and the polypodane derivative 564, respectively.303 The structures of the aglycones 563 and 564 were established by X-ray analysis. Lansium domesticum is the source of the onocerane derivatives lamesticumin A 565 and the corresponding ethyl ester 566, lamesticumins B–E 567–570, the 3-ethyl ester of lansic acid571 and ethyl lansiolate 572 together the polypodane derivative lamesticumin F 573.304 Other onocerane derivatives include cupacinoxepin574, from Cupania cinerea305 and kokosanolide B 575 and onocera-7,14-diene-3,21-dione576 from Lansium domesticum cv. Kokossan.126The isomalabaricane triterpenoids stelliferins J–N 577–581 are constituents of the sponge Rhabdastrella cf. globostellata.306 Stelliferins L–N 579–581 have a cyclised side-chain similar to rhabdastins D–G. A sponge of the genus Lipastrotethya is the source of pouosides F–I 582–585 and pouogenins A–E 586–590.307 Three iridal triterpenoids591–593 have been isolated from Iris delavayi.308
10 References
- M. B. Sporn, K. T. Liby, M. M. Yore, L. Fu, J. M. Lopchuk and G. W. Gribble, J. Nat. Prod., 2011, 74, 537–545 CrossRef CAS.
- A. Braca, P. F. Dal, S. Marzocco, G. Autore, A. Vassallo and T. N. De, Curr. Drug Targets, 2011, 12, 302–321 CrossRef CAS.
- Z. Meng, N.-g. Li, L. Zhang and A.-w. Ding, Zhonghua Zhongyiyao Xuekan, 2011, 29, 1152–1154 CAS.
- Y. Zhang, X. Zhang and G. Chen, Hainan Shifan Daxue Xuebao, Ziran Kexueban, 2011, 24, 92–95 CAS.
- M. E. Juan and J. M. Planas, Bioact. Foods Extr., 2011, 403–413 CAS.
- A. Bishayee, S. Ahmed, N. Brankov and M. Perloff, Front. Biosci., 2011, 16, 980–996 CrossRef CAS.
- B. K. Cassels and M. Asencio, Phytochem. Rev., 2011, 10, 545–564 CrossRef CAS.
- A. Gonzalez-Coloma, C. Lopez-Balboa, O. Santana, M. Reina and B. M. Fraga, Phytochem. Rev., 2011, 10, 245–260 CrossRef CAS.
- H. Sheng and H. Sun, Nat. Prod. Rep., 2011, 28, 543–593 RSC.
- Y.-X. Sun, J.-C. Liu and D.-Y. Liu, Pharmazie, 2011, 66, 813–821 CAS.
- X.-s. Zhang and X.-m. Hu, Anhui Nongye Kexue, 2011, 39, 817–818 CAS , 825.
- H. Hussain, J. Hussain, A. Al-Harrasi and Z. K. Shinwari, Pak. J. Bot., 2011, 43, 51–62 CAS.
- L. F. Barbosa, R. Braz-Filho and I. J. C. Vieira, Chem. Biodiversity, 2011, 8, 2163–2178 CAS.
- T. P. Kukina and E. N. Shmidt, Khim. Interesakh Ustoich. Razvit., 2011, 19, 655–659 CAS.
- Q.-G. Tan and X.-D. Luo, Chem. Rev., 2011, 111, 7437–7522 CrossRef CAS.
- A. Osbourn, R. J. M. Goss and R. A. Field, Nat. Prod. Rep., 2011, 28, 1261–1268 RSC.
- J. M. Augustin, V. Kuzina, S. B. Andersen and S. Bak, Phytochemistry, 2011, 72, 435–457 CrossRef CAS.
- E. Lambert, A. Faizal and D. Geelen, Appl. Biochem. Biotechnol., 2011, 164, 220–237 CrossRef CAS.
- P. Zhao, D.-F. Gao, M. Xu, Z.-G. Shi, D. Wang, C.-R. Yang and Y.-J. Zhang, Chem. Biodiversity, 2011, 8, 1931–1942 CAS.
- J. Ni, M. Chen and Y. Lin, Zhongyaocai, 2011, 34, 317–319 CAS.
- X. Xia, C. Zheng, C. Feng and C. Xia, Chaye Kexue, 2011, 31, 391–398 CAS.
- S. Boettger and M. F. Melzig, Phytochem. Lett., 2011, 4, 59–68 CrossRef CAS.
- F. Cen-Pacheco, J. A. Villa-Pulgarin, F. Mollinedo, M. Norte, A. H. Daranas and J. J. Fernández, Eur. J. Med. Chem., 2011, 46, 3302–3308 CrossRef CAS.
- F. Cen-Pacheco, J. A. Villa-Pulgarin, F. Mollinedo, M. N. Martin, J. J. Fernández and A. H. Daranas, Mar. Drugs, 2011, 9, 2220–2235 CrossRef.
- R. C. Jadulco, M. Koch, W. R. M. Van, C. Pond, O. G. Gideon, T. Matainaho, P. Piskaut and L. R. Barrows, Planta Med., 2011, 77, 1651–1654 CrossRef CAS.
- M. Spanova and G. Daum, Eur. J. Lipid Sci. Technol., 2011, 113, 1299–1320 CrossRef CAS.
- F.-Y. Meng, J.-X. Sun, X. Li, H.-F. Pi, P. Zhang and H.-L. Ruan, Helv. Chim. Acta, 2011, 94, 1778–1785 CrossRef CAS.
- S. Dall'Acqua, P. Minesso, S. Comai, B. B. Shrestha, M. B. Gewali, P. K. Jha and G. Innocenti, Nat. Prod. Commun., 2011, 6, 793–798 CAS.
- D.-W. Ou-Yang, L. Wu, Y.-L. Li, P.-M. Yang, D.-Y. Kong, X.-W. Yang and W.-D. Zhang, Phytochemistry, 2011, 72, 2197–2204 CrossRef CAS.
- A. Tsopmo and P. Kamnaing, Phytochem. Lett., 2011, 4, 218–221 CrossRef CAS.
- T.-G. Cai and Y. Cai, Chem. Biodiversity, 2011, 8, 2135–2143 CAS.
- X.-Q. Zhang, F. C. F. Ip, D.-M. Zhang, L.-X. Chen, W. Zhang, Y.-L. Li, N. Y. Ip and W.-C. Ye, Nat. Prod. Res., 2011, 25, 1607–1613 CrossRef CAS.
- I. S. Lee, B. R. Ahn, J. S. Choi, M. Hattori, B. S. Min and K. H. Bae, Bioorg. Med. Chem. Lett., 2011, 21, 6603–6607 CrossRef CAS.
- S. Joseph, K. K. Janardhanan, V. George and S. Baby, Phytochem. Lett., 2011, 4, 386–388 CrossRef CAS.
- J.-L. Rios, Planta Med., 2011, 77, 681–691 CrossRef CAS.
- M. R. Camargo and R. Kaneno, Annu. Rev. Biomed. Eng., 2011, 13, 1–8 CrossRef CAS.
- X.-W. Shi, X.-J. Li, J.-M. Gao and X.-C. Zhang, Chem. Biodiversity, 2011, 8, 1864–1870 CAS.
- M. Isaka, P. Chinthanom, M. Sappan, R. Chanthaket, J. J. Luangsa-ard, S. Prabpai and P. Kongsaeree, J. Nat. Prod., 2011, 74, 2143–2150 CrossRef CAS.
- T. Lin, X. Lin, C.-H. Lu and Y.-M. Shen, Helv. Chim. Acta, 2011, 94, 301–305 CrossRef CAS.
- R. Tanaka, M. Toyoshima and T. Yamada, Phytochem. Lett., 2011, 4, 328–332 CrossRef CAS.
- A. S. Antonov, A. I. Kalinovsky, P. S. Dmitrenok, V. I. Kalinin, V. A. Stonik, E. Mollo and G. Cimino, Carbohydr. Res., 2011, 346, 2182–2192 CrossRef CAS.
- M. Ono, D. Toyohisa, T. Morishita, H. Horita, S. Yasuda, Y. Nishida, T. Tanaka, M. Okawa, J. Kinjo, H. Yoshimitsu and T. Nohara, Chem. Pharm. Bull., 2011, 59, 1348–1354 CrossRef CAS.
- Z. Jabbar and M. Ali, Int. Res. J. Pharm., 2011, 2(7), 141–144 CAS.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko and V. I. Kalinin, Nat. Prod. Commun., 2011, 6, 1075–1082 CAS.
- V. P. Careaga, C. Muniain and M. S. Maier, Chem. Biodiversity, 2011, 8, 467–475 CAS.
- A. S. Antonov, S. A. Avilov, A. I. Kalinovsky, P. S. Dmitrenok, V. I. Kalinin, S. Taboada, M. Ballesteros and C. Avila, Nat. Prod. Res., 2011, 25, 1324–1333 CrossRef CAS.
- Y.-B. Xue, J.-H. Yang, X.-N. Li, X. Du, J.-X. Pu, W.-L. Xiao, J. Su, W. Zhao, Y. Li and H.-D. Sun, Org. Lett., 2011, 13, 1564–1567 CrossRef CAS.
- Y.-C. Lin, I. W. Lo, S.-Y. Chen, P.-H. Lin, C.-T. Chien, S.-y. Chang and Y.-C. Shen, Org. Lett., 2011, 13, 446–449 CrossRef CAS.
- F.-Y. Meng, J.-X. Sun, X. Li, H.-Y. Yu, S.-M. Li and H.-L. Ruan, Org. Lett., 2011, 13, 1502–1505 CrossRef CAS.
- J.-R. Wang, Z.-B. Zhao and Y.-W. Guo, J. Asian Nat. Prod. Res., 2011, 13, 551–555 CrossRef CAS.
- Y. Jiang, G.-Z. Yang, Y. Chen, M.-C. Liao, X.-M. Liu, S. Chen, L. Liu and X.-X. Lei, Helv. Chim. Acta, 2011, 94, 491–496 CrossRef CAS.
- G.-Y. Yang, Y.-K. Li, X.-J. Zhang, X.-N. Li, L.-M. Yang, Y.-M. Shi, W.-L. Xiao, Y.-T. Zheng and H.-D. Sun, Nat. Prod. Bioprospect., 2011, 1, 33–36 CrossRef CAS.
- J.-M. Tan, Y.-H. Qiu, X.-Q. Tan and C.-H. Tan, Helv. Chim. Acta, 2011, 94, 1697–1702 CrossRef CAS.
- W.-J. He, H.-B. Chu, Y.-M. Zhang, H.-J. Han, H. Yan, G.-Z. Zeng, Z.-H. Fu, O. Olubanke and N.-H. Tan, Planta Med., 2011, 77, 1924–1931 CrossRef CAS.
- H.-X. Kuang, Q.-H. Wang, B.-Y. Yang, Z.-B. Wang, Y. Okada and T. Okuyama, Helv. Chim. Acta, 2011, 94, 2239–2247 CrossRef CAS.
- M. K. Jamroz, M. H. Jamroz, J. C. Dobrowolski, J. A. Glinski, M. H. Davey and I. Wawer, Spectrochim. Acta, Part A, 2011, 78A, 107–112 CrossRef CAS.
- Y. Ippongi, T. Ohtsuki, K. Toume, M. A. Arai, Y. Yamamoto and M. Ishibashi, Chem. Pharm. Bull., 2011, 59, 279–281 CrossRef CAS.
- Y. Kashiwada, K. Nishimura, S.-i. Kurimoto and Y. Takaishi, Bioorg. Med. Chem., 2011, 19, 2790–2796 CrossRef CAS.
- T. Nuanyai, R. Sappapan, T. Vilaivan and K. Pudhom, Phytochem. Lett., 2011, 4, 26–29 CrossRef CAS.
- Y. Shu, L. Cheng and P. Yang, Zhongcaoyao, 2011, 42, 805–813 CAS.
- H.-T. Kuo, C.-F. Peng, H.-Y. Huang, C.-H. Lin, I.-S. Chen and I.-L. Tsai, Planta Med., 2011, 77, 736–741 CrossRef CAS.
- T. Nuanyai, R. Sappapan, T. Vilaivan and K. Pudhom, Chem. Pharm. Bull., 2011, 59, 385–387 CrossRef CAS.
- R. Sun, H.-C. Song, C.-R. Wang, K.-Z. Shen, Y.-B. Xu, Y.-X. Gao, Y.-G. Chen and J.-Y. Dong, Bioorg. Med. Chem. Lett., 2011, 21, 961–965 CrossRef CAS.
- Y. Nian, X.-M. Zhang, Y. Li, Y.-Y. Wang, J.-C. Chen, L. Lu, L. Zhou and M.-H. Qiu, Phytochemistry, 2011, 72, 1473–1481 CrossRef CAS.
- D.-S. Li, Y. Nian, Y. Sun and M.-H. Qiu, Helv. Chim. Acta, 2011, 94, 632–638 CrossRef CAS.
- M. Nishida and H. Yoshimitsu, Chem. Pharm. Bull., 2011, 59, 1243–1249 CrossRef CAS.
- Z. Zhao, K. Matsunami, H. Otsuka, T. Shinzato and Y. Takeda, Chem. Pharm. Bull., 2011, 59, 902–905 CrossRef CAS.
- H. D. Nguyen, B. T. D. Trinh, Q. N. Tran, H. D. Nguyen, H. D. Pham, P. E. Hansen, F. Duus, J. D. Connolly and L.-H. D. Nguyen, Phytochemistry, 2011, 72, 290–295 CrossRef CAS.
- K. Toume, T. Nakazawa, T. Ohtsuki, M. A. Arai, T. Koyano, T. Kowithayakorn and M. Ishibashi, J. Nat. Prod., 2011, 74, 249–255 CrossRef CAS.
- S. Jan, A. Abbaskhan, S. G. Musharraf, S. A. Sattar, Samreen, S. I. Resayes, Z. A. Al-Othman, A. M. Al-Majid, R. Attaur and M. I. Choudhary, Planta Med., 2011, 77, 1829–1834 CrossRef CAS.
- H. N. Nguyen, P. V. Kiem, N. K. Ban, P. T. Nguyen, X. N. Nguyen, X. C. Nguyen, C. Tistaert, B. Dejaegher, H. Y. Vander, J. Quetin-Leclercq, D. T. Thao and M. C. Van, Phytochem. Lett., 2011, 4, 348–352 CAS.
- Y. Qiang, J.-M. Ni, Y.-B. Shi, X.-Y. Zhang, X. Yang and S.-T. Li, J. Chem. Res., 2011, 35, 664–665 CrossRef CAS.
- N. B. Truong, C. V. Pham, H. T. M. Doan, H. V. Nguyen, C. M. Nguyen, H. T. Nguyen, H.-j. Zhang, H. H. S. Fong, S. G. Franzblau, D. D. Soejarto and M. V. Chau, J. Nat. Prod., 2011, 74, 1318–1322 CrossRef CAS.
- D. Wakana, N. Kawahara and Y. Goda, J. Nat. Med., 2011, 65, 18–23 CrossRef CAS.
- I. M. Isaev and M. I. Isaev, Chem. Nat. Compd., 2011, 47, 587–591 CrossRef CAS.
- J. Linnek, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, T. Paululat, S. Avunduk, Ö. Alankuş-Çalişkan and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2011, 94, 230–237 CrossRef CAS.
- H. Kuang, Y. Su, B. Yang, Y. Xia, Q. Wang, Z. Wang and Z. Yu, Molecules, 2011, 16, 4348–4357 CrossRef CAS.
- T. K. Naubeev, K. K. Uteniyazov and M. I. Isaev, Chem. Nat. Compd., 2011, 47, 250–253 CrossRef CAS.
- Z. Tian, Y. Sun, P. Xiao and E. Wu, Recent Prog. Med. Plants, 2011, 31, 49–63 CAS.
- M. Liu, M. Gan, S. Lin, Y. Zhang, J. Zi, W. Song, X. Fan, Y. Liu, Y. Yang and J. Shi, Org. Lett., 2011, 13, 2856–2859 CrossRef CAS.
- M. Gan, M. Liu, B. Liu, S. Lin, Y. Zhang, J. Zi, W. Song, F. Ye, X. Chen and J. Shi, J. Nat. Prod., 2011, 74, 2431–2437 CrossRef CAS.
- K. L. Lang, T. de R. Guimarães, V. R. Machado, L. A. Zimmermann, I. T. Silva, M. R. Teixeira, F. J. Duran, J. A. Falermo, C. M. O. Simões, M. S. B. Caro and E. P. Schenkel, Planta Med., 2011, 77, 1648–1651 CrossRef CAS.
- K.-W. Lin, S.-C. Yang and C.-N. Lin, Food Chem., 2011, 127, 609–614 CrossRef CAS.
- C. Hsu, C.-L. Hsieh, Y.-H. Kuo and C.-j. Huang, J. Agric. Food Chem., 2011, 59, 4553–4561 CrossRef CAS.
- J.-Q. Cao, Y. Zhang, J.-M. Cui and Y.-Q. Zhao, Chin. Chem. Lett., 2011, 22, 583–586 CrossRef CAS.
- H. M. Ekramul, A. M. Badrul and H. M. Sarowar, Int. J. Pharm. Sci. Res., 2011, 2, 1135–1146 Search PubMed.
- N. Li, C.-F. Wu, X.-Y. Xu, Z.-Y. Liu, X. Li and Y.-Q. Zhao, Eur. J. Med. Chem., 2012, 50, 173–178 CrossRef CAS.
- X. Li, J. Q. Cao, L. Shi and Y. Q. Zhao, Chin. Chem. Lett., 2011, 22, 1461–1464 CrossRef CAS.
- R. Grougnet, P. Magiatis, S. Mitaku, A.-L. Skaltsounis, P. Cabalion, F. Tillequin and S. Michel, Helv. Chim. Acta, 2011, 94, 656–661 CrossRef CAS.
- J.-M. Zhao, N. Li, H. Zhang, C.-f. Wu, H.-R. Piao and Y.-Q. Zhao, Bioorg. Med. Chem. Lett., 2011, 21, 1027–1031 CrossRef CAS.
- J. Xiong, M. Taniguchi, Y. Kashiwada, T. Yamagishi and Y. Takaishi, J. Nat. Med., 2011, 65, 217–223 CrossRef CAS.
- T. Nuanyai, R. Sappapan, T. Vilaivan and K. Pudhom, Phytochem. Lett., 2011, 4, 183–186 CrossRef CAS.
- T. Asai and Y. Fujimoto, Phytochem. Lett., 2011, 4, 38–42 CrossRef CAS.
- W. Ding, F. Zeng, L. Xu, Y. Chen, Y. Wang and X. Wei, J. Nat. Prod., 2011, 74, 1868–1874 CrossRef CAS.
- G.-Y. Zhu, Y.-W. Li, D. K.-P. Hau, Z.-H. Jiang, Z.-L. Yu and W.-F. Fong, J. Agric. Food Chem., 2011, 59, 200–205 CrossRef CAS.
- H.-H. Chan, T.-L. Hwang, M. V. B. Reddy, D.-T. Li, K. Qian, K. F. Bastow, K.-H. Lee and T.-S. Wu, J. Nat. Prod., 2011, 74, 796–802 CrossRef CAS.
- L.-W. Qi, C.-Z. Wang and C.-S. Yuan, Phytochemistry, 2011, 72, 689–699 CrossRef CAS.
- K. Radad, R. Moldzio and W.-D. Rausch, CNS Neurosci. Ther., 2011, 17, 761–767 CrossRef CAS.
- M.-G. Phan, T.-T. C. Truong, T.-S. Phan, K. Matsunami and H. Otsuka, Phytochem. Lett., 2011, 4, 179–182 CrossRef CAS.
- X.-X. Weng, Y. Shao, Y.-Y. Chen, W. Gao, L. Cheng and D.-Y. Kong, J. Asian Nat. Prod. Res., 2011, 13, 749–755 CrossRef CAS.
- G.-Y. Zhu, Y.-W. Li, D. K.-P. Hau, Z.-H. Jiang, Z.-L. Yu and W.-F. Fong, Chem. Biodiversity, 2011, 8, 1853–1863 CAS.
- S.-J. Qu, J.-J. Tan, J.-G. Cai, Y.-P. Ling, S.-F. Zhang, C.-H. Tan and D.-Y. Zhu, J. Asian Nat. Prod. Res., 2011, 13, 178–181 CrossRef CAS.
- J. H. Kim and Y. N. Han, Phytochemistry, 2011, 72, 1453–1459 CrossRef CAS.
- Q. Liu, J.-J. Lv, M. Xu, D. Wang, H.-T. Zhu, C.-R. Yang and Y.-J. Zhang, Nat. Prod. Bioprospect., 2011, 1, 124–128 CrossRef CAS.
- J.-P. Liu, D. Lu and P.-Y. Li, J. Asian Nat. Prod. Res., 2011, 13, 198–204 CrossRef CAS.
- M. Zhou, M. Xu, D. Wang, H.-T. Zhu, C.-R. Yang and Y.-J. Zhang, Helv. Chim. Acta, 2011, 94, 2010–2019 CrossRef CAS.
- L. Shi, J.-Q. Cao, S.-M. Shi and Y.-Q. Zhao, J. Asian Nat. Prod. Res., 2011, 13, 168–177 CrossRef CAS.
- J.-R. Wang, H.-L. Liu, T. Kurtán, A. Mándi, S. Antus, J. Li, H.-Y. Zhang and Y.-W. Guo, Org. Biomol. Chem., 2011, 9, 7685–7696 CAS.
- J. Hu, X. Wang and X. Shi, Eur. J. Org. Chem., 2011, 2011, 7215–7223 CrossRef CAS.
- Y. Zhang, J.-S. Wang, J. Luo and L.-Y. Kong, Chem. Pharm. Bull., 2011, 59, 282–286 CrossRef CAS.
- H.-L. Huang, C.-M. Wang, Z.-H. Wang, M.-J. Yao, G.-T. Han, J.-C. Yuan, K. Gao and C.-S. Yuan, J. Nat. Prod., 2011, 74, 2235–2242 CrossRef CAS.
- J. Wang, Y. Zhang, J. Luo and L. Kong, Magn. Reson. Chem., 2011, 49, 450–457 CrossRef CAS.
- J.-S. Wang, Y. Zhang, D.-D. Wei, X.-B. Wang, J. Luo and L.-Y. Kong, Chem. Biodiversity, 2011, 8, 2025–2034 CAS.
- K. H. Kim, S. U. Choi, Y. C. Kim and K. R. Lee, J. Nat. Prod., 2011, 74, 54–59 CrossRef CAS.
- X.-J. Zhou, M. Xu, X.-S. Li, Y.-H. Wang, Y. Gao, R. Cai and Y.-X. Cheng, Bull. Korean Chem. Soc., 2011, 32, 127–130 CrossRef CAS.
- F. Wang, Z.-L. Li, H.-H. Cui, H.-M. Hua, Y.-K. Jing and S.-W. Liang, J. Asian Nat. Prod. Res., 2011, 13, 193–197 CrossRef CAS.
- S. A. Shaheen, Z. M. H. Abu, I. K. Nazer, R. M. Darwish and H. I. Al-Jaber, Nat. Prod. Res., 2011, 25, 1312–1318 CrossRef CAS.
- J. X. Chen, J. C. Chen, Y. Sun, Y. X. Yan, L. M. Kong, Y. Li and M. H. Qiu, Planta Med., 2011, 77, 1844–1847 CrossRef CAS.
- H.-T. Zhong, F. Li, B. Chen and M.-K. Wang, Helv. Chim. Acta, 2011, 94, 2061–2065 CrossRef CAS.
- F. U. Khan, J. Hussain, I. U. Khan, R. Ullah, I. Ali, Z. Muhammad, H. Hussain and M. R. Shah, Chem. Nat. Compd., 2011, 47, 234–236 CrossRef CAS.
- S.-B. Wu, Q.-Y. Bao, W.-X. Wang, Y. Zhao, G. Xia, Z. Zhao, H. Zeng and J.-F. Hu, Planta Med., 2011, 77, 922–928 CrossRef CAS.
- S.-i. Kurimoto, Y. Kashiwada, K.-H. Lee and Y. Takaishi, Phytochemistry, 2011, 72, 2205–2211 CrossRef CAS.
- X. Fang, Y. T. Di and X. J. Hao, Curr. Org. Chem., 2011, 15, 1363–1391 CrossRef CAS.
- B. M. Komane, E. I. Olivier and A. M. Viljoen, Phytochem. Lett., 2011, 4, 1–9 CrossRef CAS.
- B. Heasley, Eur. J. Org. Chem., 2011, 19–46 CrossRef CAS.
- T. Mayanti, R. Tjokronegoro, U. Supratman, M. R. Mukhtar, K. Awang and A. H. A. Hadi, Molecules, 2011, 16, 2785–2795 CrossRef CAS.
- I. A. Najmuldeen, A. H. A. Hadi, K. Awang, K. Mohamad, K. A. Ketuly, M. R. Mukhtar, S.-L. Chong, G. Chan, M. A. Nafiah, N. S. Weng, O. Shirota, T. Hosoya, A. E. Nugroho and H. Morita, J. Nat. Prod., 2011, 74, 1313–1317 CrossRef CAS.
- X.-H. Yan, Y.-T. Di, X. Fang, S.-Y. Yang, H.-P. He, S.-L. Li, Y. Lu and X.-J. Hao, Phytochemistry, 2011, 72, 508–513 CrossRef CAS.
- Y. Zhang, J.-S. Wang, X.-B. Wang, D.-D. Wei, J.-G. Luo, J. Luo, M.-H. Yang and L.-Y. Kong, Tetrahedron Lett., 2011, 52, 2590–2593 CrossRef CAS.
- S.-P. Yang, H.-D. Chen, S.-G. Liao, B.-J. Xie, Z.-H. Miao and J.-M. Yue, Org. Lett., 2011, 13, 150–153 CrossRef CAS.
- C. P. Wong, M. Shimada, Y. Nagakura, A. E. Nugroho, Y. Hirasawa, T. Kaneda, K. Awang, A. H. A. Hadi, K. Mohamad, M. Shiro and H. Morita, Chem. Pharm. Bull., 2011, 59, 407–411 CrossRef CAS.
- Z.-S. Su, S.-P. Yang, S. Zhang, L. Dong and J.-M. Yue, Helv. Chim. Acta, 2011, 94, 1515–1526 CrossRef CAS.
- J.-L. Yang, L.-L. Liu and Y.-P. Shi, Planta Med., 2011, 77, 271–276 CrossRef CAS.
- C. A. C. Barrera, E. D. C. Barrera, D. S. G. Falla, G. D. Murcia and L. E. C. Suarez, Chem. Pharm. Bull., 2011, 59, 855–859 CrossRef CAS.
- J. Liu, S.-P. Yang, Z.-S. Su, B.-D. Lin, Y. Wu and J.-M. Yue, Phytochemistry, 2011, 72, 2189–2196 CrossRef CAS.
- F. Zhang, S.-G. Liao, C.-R. Zhang, X.-F. He, W.-S. Chen and J.-M. Yue, Planta Med., 2011, 77, 1617–1622 CrossRef CAS.
- T. K. Nsiama, H. Okamura, T. Hamada, Y. Morimoto, M. Doe, T. Iwagawa and M. Nakatani, Phytochemistry, 2011, 72, 1854–1858 CrossRef CAS.
- W. Ravangpai, D. Sommit, T. Teerawatananond, N. Sinpranee, T. Palaga, S. Pengpreecha, N. Muangsin and K. Pudhom, Bioorg. Med. Chem. Lett., 2011, 21, 4485–4489 CrossRef CAS.
- J.-F. Hu, H. Fan, L.-J. Wang, S.-B. Wu and Y. Zhao, Phytochem. Lett., 2011, 4, 292–297 CrossRef CAS.
- H.-B. Liu, C.-R. Zhang, S.-H. Dong, L. Dong, Y. Wu and J.-M. Yue, Chem. Pharm. Bull., 2011, 59, 1003–1007 CrossRef CAS.
- J. Luo, J.-S. Wang, J.-G. Luo, X.-B. Wang and L.-Y. Kong, Tetrahedron, 2011, 67, 2942–2948 CrossRef CAS.
- J. Luo, J.-S. Wang, X.-B. Wang, J.-G. Luo and L.-Y. Kong, Chem. Pharm. Bull., 2011, 59, 225–230 CrossRef CAS.
- J. Luo, Y. Li, J.-S. Wang and L.-Y. Kong, Chem. Biodiversity, 2011, 8, 2261–2269 CAS.
- Y. Li, J. Luo, Q. Wang and L.-Y. Kong, J. Asian Nat. Prod. Res., 2011, 13, 781–786 CrossRef CAS.
- J.-L. Yin, Y.-T. Di, X. Fang, E.-D. Liu, H.-Y. Liu, H.-P. He, S.-L. Li, S.-F. Li and X.-J. Hao, Tetrahedron Lett., 2011, 52, 3083–3085 CrossRef CAS.
- J. Luo, J.-S. Wang, W.-J. Cao and L.-Y. Kong, Zhongguo Tianran Yaowu, 2011, 9, 98–100 CAS.
- B.-D. Lin, C.-R. Zhang, S.-P. Yang, Y. Wu and J.-M. Yue, Chem. Pharm. Bull., 2011, 59, 458–465 CrossRef CAS.
- Q. Zhang, Y.-T. Di, H.-P. He, X. Fang, D.-L. Chen, X.-H. Yan, F. Zhu, T.-Q. Yang, L.-L. Liu and X.-J. Hao, J. Nat. Prod., 2011, 74, 152–157 CrossRef CAS.
- Y. Tanaka, T. Yamada, Y. In, O. Muraoka, T. Kajimoto and R. Tanaka, Tetrahedron, 2011, 67, 782–792 CrossRef CAS.
- J. Li, M.-Y. Li, T. Satyanandamurty and J. Wu, Helv. Chim. Acta, 2011, 94, 1651–1656 CrossRef CAS.
- W.-H. Jiao, H. Gao, F. Zhao, F. He, G.-X. Zhou and X.-S. Yao, Chem. Biodiversity, 2011, 8, 1163–1169 CAS.
- M. Zhao, S. T. Lau, X. Q. Zhang, W. C. Ye, P. S. Leung, C.-T. Che and Z.-X. Lin, Helv. Chim. Acta, 2011, 94, 2099–2105 CrossRef CAS.
- H. Chen, J. Bai, Z.-F. Fang, S.-S. Yu, S.-G. Ma, S. Xu, Y. Li, J. Qu, J.-H. Ren, L. Li, Y.-K. Si and X.-G. Chen, J. Nat. Prod., 2011, 74, 2438–2445 CrossRef CAS.
- T. Diyabalanage, R. Ratnayake, J. A. Wilson, C. J. Henrich, J. A. Beutler, N. H. Colburn, J. B. McMahon and K. R. Gustafson, Bioorg. Med. Chem. Lett., 2011, 21, 4397–4399 CrossRef CAS.
- H. R. Siddique and M. Saleem, Life Sci., 2011, 88, 285–293 CrossRef CAS.
- C. Gauthier, J. Legault, M. Piochon-Gauthier and A. Pichette, Phytochem. Rev., 2011, 10, 521–544 CrossRef CAS.
- J. Shinozaki, T. Nakane, N. Onodera, A. Takano and K. Masuda, Chem. Pharm. Bull., 2011, 59, 767–769 CrossRef CAS.
- Y.-P. Liu, X.-H. Cai, T. Feng, Y. Li, X.-N. Li and X.-D. Luo, J. Nat. Prod., 2011, 74, 1161–1168 CrossRef CAS.
- X.-F. He, X.-N. Wang, S. Yin, L. Dong and J.-M. Yue, Bioorg. Med. Chem. Lett., 2011, 21, 125–129 CrossRef CAS.
- N.-Y. Yang, J.-H. Chen, G.-S. Zhou, Y.-P. Tang, J.-A. Duan, L.-J. Tian and X.-H. Liu, Fitoterapia, 2011, 82, 927–931 CrossRef CAS.
- M. H. Kazmi, I. Fatima, A. Malik, L. Iqbal, M. Latif and N. Afza, J. Asian Nat. Prod. Res., 2011, 13, 1081–1086 CrossRef CAS.
- T. Morikawa, H. Oominami, H. Matsuda and M. Yoshikawa, J. Nat. Med., 2011, 65, 129–134 CrossRef CAS.
- Y.-E. Guo, L.-L. Wang, Z.-L. Li, S.-L. Niu, X.-Q. Liu, H.-M. Hua, H. Chen, J. Chu and T.-C. Zhang, J. Asian Nat. Prod. Res., 2011, 13, 440–443 CrossRef CAS.
- X. Wang, E. Habib, F. Leon, M. M. Radwan, N. Tabanca, J. Gao, D. E. Wedge and S. J. Cutler, Chem. Biodiversity, 2011, 8, 2331–2340 CAS.
- X.-L. Wang, A.-E. Hay, A. Matheeussen, M. P. Gupta and K. Hostettmann, Magn. Reson. Chem., 2011, 49, 184–189 CrossRef CAS.
- S. Zhao, Z. Huang and J. Gao, Bull. Korean Chem. Soc., 2011, 32, 1368–1370 CrossRef CAS.
- Z. Shu, Z. Chen, X.-j. Ding, B.-q. Lu, C.-j. Ji, Q.-m. Xu, X.-r. Li and S.-l. Yang, Heterocycles, 2011, 83, 2365–2372 CrossRef CAS.
- P. Pailee, V. Prachyawarakorn, C. Mahidol, S. Ruchirawat and P. Kittakoop, Eur. J. Org. Chem., 2011, 2011, 3809–3814 CrossRef CAS.
- M. Lee, M. K. Lee, Y. C. Kim and S. H. Sung, Bioorg. Med. Chem. Lett., 2011, 21, 2906–2910 CrossRef CAS.
- S. V. Fannang, V. Kuete, C. D. Mbazoa, J. I. Momo, H. T. Van-Dufat, F. Tillequin, E. Seguin, E. Chosson and J. Wandji, Chem. Nat. Compd., 2011, 47, 404–407 CrossRef CAS.
- A. Yokosuka, S. Kawakami, M. Haraguchi and Y. Mimaki, Phytochem. Lett., 2011, 4, 259–266 CrossRef CAS.
- R. A. S. Macahig, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2011, 59, 397–401 CrossRef CAS.
- D. N. Vedernikov and V. I. Roshchin, Khim. Rastit. Syr'ya, 2011, 95–102 CAS.
- T. H. Quang, T. T. N. Nguyen, C. V. Minh, P. V. Kiem, X. N. Nguyen, B. H. Tai, P. T. Nguyen, H. T. Nguyen, S. B. Song and Y. H. Kim, J. Nat. Prod., 2011, 74, 1908–1915 CrossRef CAS.
- B. K. Ponou, R. B. Teponno, M. Ricciutelli, T. B. Nguelefack, L. Quassinti, M. Bramucci, G. Lupidi, L. Barboni and L. A. Tapondjou, Chem. Biodiversity, 2011, 8, 1301–1309 CAS.
- R. Badoni, D. K. Semwal, U. Rawat and M. S. M. Rawat, Helv. Chim. Acta, 2011, 94, 464–473 CrossRef CAS.
- R. B. Semwal, D. K. Semwal, R. Semwal, R. Singh and M. S. M. Rawat, J. Ethnopharmacol., 2011, 135, 78–87 CrossRef CAS.
- T. Mencherini, P. Picerno, M. Festa, P. Russo, A. Capasso and R. Aquino, J. Nat. Prod., 2011, 74, 2116–2121 CrossRef CAS.
- Y. Qu, J. Liang and X. Feng, Tianran Chanwu Yanjiu Yu Kaifa, 2011, 23, 577–581 CAS.
- Z. Zhang, C. Zhao, S. Chen and H. Ji, Yaoxue Jinzhan, 2011, 35, 353–359 CAS.
- S.-Y. Cheng, C.-M. Wang, Y.-M. Hsu, T.-J. Huang, S.-C. Chou, E.-H. Lin and C.-H. Chou, J. Nat. Prod., 2011, 74, 1744–1750 CrossRef CAS , 2030.
- D.-Q. Luo, H.-Y. Deng, X.-L. Yang, B.-Z. Shi and J.-Z. Zhang, Helv. Chim. Acta, 2011, 94, 1041–1047 CrossRef CAS.
- X.-F. Niu, X. Liu, L. Pan and L. Qi, Fitoterapia, 2011, 82, 960–963 CrossRef CAS.
- B. M. Mba'ning, B. N. Lenta, S. Ngouela, D. T. Noungoue, F. Tantangmo, F. M. Talontsi, E. Tsamo and H. Laatsch, Z. Naturforsch., B: J. Chem. Sci., 2011, 66, 1270–1274 CrossRef CAS.
- P. Liu, P. Hu, R.-X. Deng, R. Li, L. Yang and W.-P. Yin, Helv. Chim. Acta, 2011, 94, 136–141 CrossRef CAS.
- C. Y. Ragasa, D. L. Espineli and C.-C. Shen, Chem. Pharm. Bull., 2011, 59, 778–782 CrossRef CAS.
- X.-Q. Chen, Y. Li, J. He, X. Cheng, K. Wang, M.-M. Li, Z.-H. Pan, L.-Y. Peng and Q.-S. Zhao, Chem. Pharm. Bull., 2011, 59, 496–498 CrossRef CAS.
- N. Zeng, Y. Shen, L.-Z. Li, W.-H. Jiao, P.-Y. Gao, S.-J. Song, W.-S. Chen and H.-W. Lin, J. Nat. Prod., 2011, 74, 732–738 CrossRef CAS.
- H. Yang, E. J. Jeong, J. Kim, S. H. Sung and Y. C. Kim, J. Nat. Prod., 2011, 74, 751–756 CrossRef CAS.
- C.-B. Xue, D.-W. Chai, X.-J. Jin, Y.-R. Bi, X.-J. Yao, W.-S. Wu and Y. Zhu, Phytochemistry, 2011, 72, 1804–1813 CrossRef CAS.
- M. Shaaban, K. A. Shaaban, H. I. Abd-Alla, A. G. Hanna and H. Laatsch, Z. Naturforsch., B: J. Chem. Sci., 2011, 66, 425–432 CrossRef CAS.
- Y.-L. Huang, T. Tsujita, T. Tanaka, Y. Matsuo, I. Kouno, D.-P. Li and G.-i. Nonaka, Phytochemistry, 2011, 72, 2006–2014 CrossRef CAS.
- X.-Q. Liu, H.-L. Huang, M.-J. Yao, G.-T. Han, N. Liu, J.-C. Yuan and C.-S. Yuan, Helv. Chim. Acta, 2011, 94, 2264–2271 CrossRef CAS.
- M. J. Nunez, M. L. Kennedy, I. A. Jimenez and I. L. Bazzocchi, Tetrahedron, 2011, 67, 3030–3033 CrossRef CAS.
- J.-G. Luo, W. Nie and L.-Y. Kong, J. Asian Nat. Prod. Res., 2011, 13, 529–533 CrossRef CAS.
- D. Liang, Z.-Y. Hao, G.-J. Zhang, Q.-J. Zhang, R.-Y. Chen and D.-Q. Yu, J. Nat. Prod., 2011, 74, 2128–2136 CrossRef CAS.
- X.-A. Huang, X.-L. Shen, Y.-J. Hu, Y.-M. Liu, K.-L. Liu, F.-X. Zhang and X.-X. Zhou, Molecules, 2011, 16, 8076–8082 CrossRef CAS.
- T. Varughese, M. M. Manir, M. Rahaman, J. K. Kim, B.-G. Lee and S.-S. Moon, Planta Med., 2011, 77, 2029–2036 CrossRef CAS.
- K. Matsunami, H. Otsuka and Y. Takeda, Chem. Pharm. Bull., 2011, 59, 1274–1280 CrossRef CAS.
- T. Nakano, S. Sugimoto, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2011, 59, 1141–1148 CrossRef CAS.
- S. Okazaki, K. Kinoshita, S. Ito, K. Koyama, H. Yuasa and K. Takahashi, Phytochemistry, 2011, 72, 136–146 CrossRef CAS.
- L. Gao, L. Zhang, N. Li, J.-Y. Liu, P.-l. Cai and S.-l. Yang, Carbohydr. Res., 2011, 346, 2881–2885 CrossRef CAS.
- L. O. A. Manguro, J. O. Midiwo, L. F. Tietze and P. Hao, ARKIVOC, 2011,(ii), 172–198 CrossRef CAS.
- Y. Nakahara, M. Okawa, J. Kinjo and T. Nohara, Chem. Pharm. Bull., 2011, 59, 1329–1339 CrossRef CAS.
- K. H. Kim, I. K. Lee, S. U. Choi, J. H. Lee, E. Moon, S. Y. Kim and K. R. Lee, Planta Med., 2011, 77, 1555–1558 CrossRef CAS.
- W. Dong, X. Liu, X. Li, D. Yang and L. Ding, Fitoterapia, 2011, 82, 782–785 CrossRef CAS.
- K. Foubert, F. Cuyckens, A. Matheeussen, A. Vlietinck, S. Apers, L. Maes and L. Pieters, Phytochemistry, 2011, 72, 1414–1423 CrossRef CAS.
- D.-L. Liu, N.-L. Wang, X. Zhang and X.-S. Yao, Helv. Chim. Acta, 2011, 94, 693–702 CrossRef CAS.
- D. Liu, X. Zhang, S. Wang, N. Wang and X. Yao, Chin. Chem. Lett., 2011, 22, 957–960 CrossRef CAS.
- A. Rubio-Moraga, G. J. Gerwig, N. Castro-Diaz, M. L. Jimeno, J. Escribano, J.-A. Fernandez and J. P. Kamerling, Ind. Crops Prod., 2011, 34, 1401–1409 CrossRef CAS.
- H. T. Nguyen, H. Q. Tran, T. T. N. Nguyen, V. M. Chau, K. A. Bui, Q. L. Pham, M. C. Nguyen and Y. H. Kim, Chem. Pharm. Bull., 2011, 59, 1417–1420 CrossRef.
- E. P. Mazzola, A. Parkinson, E. J. Kennelly, B. Coxon, L. S. Einbond and D. I. Freedberg, Carbohydr. Res., 2011, 346, 759–768 CrossRef CAS.
- G.-L. Jin, C.-J. Zheng, W.-B. Xin, Z.-J. Mao, P.-X. Sun, Z.-X. Zeng and L.-P. Qin, Arch. Pharmacal Res., 2011, 34, 869–873 CrossRef CAS.
- S. Yao, J.-G. Luo, L. Ma and L.-Y. Kong, Zhongguo Tianran Yaowu, 2011, 9, 401–405 CAS.
- G. Gao, Z. Lu, S. Tao, S. Zhang and F. Wang, Carbohydr. Res., 2011, 346, 2200–2205 CrossRef CAS.
- G.-C. Gao, Z.-X. Lu, S.-H. Tao, S. Zhang, F.-Z. Wang and Q.-X. Li, Can. J. Chem., 2011, 89, 1277–1282 CrossRef CAS.
- Q. Xue, Z.-L. Sun, M.-L. Guo, Y. Wang, G. Zhang and X.-K. Wang, Nat. Prod. Res., 2011, 25, 772–780 CrossRef CAS.
- Q. Wu, Y. Wang and M. Guo, Chem. Pharm. Bull., 2011, 59, 666–671 CrossRef CAS.
- Z. Ali and I. A. Khan, Phytochemistry, 2011, 72, 2075–2080 CrossRef CAS.
- A. F. Awantu, B. N. Lenta, T. Bogner, Y. F. Fongang, S. Ngouela, J. D. Wansi, E. Tsamo and N. Sewald, Z. Naturforsch., B: J. Chem. Sci., 2011, 66, 624–628 CrossRef CAS.
- J.-J. Liu, X.-L. Wang, B.-L. Guo, W.-H. Huang, P.-G. Xiao, C.-Q. Huang, L.-Z. Zheng, G. Zhang, L. Qin and G.-Z. Tu, J. Asian Nat. Prod. Res., 2011, 13, 851–860 CrossRef CAS.
- X. N. Nguyen, H. Y. Lim, P. V. Kiem, C. V. Minh, V. K. Thu, B. H. Tai, T. H. Quang, S. B. Song and Y. H. Kim, Bioorg. Med. Chem. Lett., 2011, 21, 6143–6147 CrossRef CAS.
- J. He, J. Ma, D.-W. Lai, Y.-m. Zhang and W.-J. Sun, Nat. Prod. Res., 2011, 25, 1771–1775 CrossRef CAS.
- H.-Z. Fu, C.-J. Li, J.-Z. Yang, Z.-F. Shen and D.-M. Zhang, J. Nat. Prod., 2011, 74, 1066–1072 CrossRef CAS.
- Z. A. Kozhamkulova, M. M. Radwan, G. E. Zhusupova, Z. A. Abilov and S. A. Ross, Phytochem. Lett., 2011, 4, 323–327 CrossRef CAS.
- T. K. Tabopda, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, J.-F. Mirjolet, O. Duchamp, B. T. Ngadjui and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2011, 94, 2066–2076 CrossRef CAS.
- Y. Chen, Y. Zhao, M. Wang, H. Sun, Y. Dong and X. Feng, Chem. Nat. Compd., 2011, 47, 940–943 CrossRef.
- Y.-X. He, L. Li, K. Zhang and Z.-R. Liu, J. Asian Nat. Prod. Res., 2011, 13, 1104–1109 CrossRef CAS.
- T. K. Tabopda, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, B. T. Ngadjui, J.-F. Mirjolet, O. Duchamp and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2011, 94, 914–922 CrossRef CAS.
- Z.-Q. Cheng, D. Yang, Q.-Y. Ma, X.-H. Yi, N.-L. Zhang, J. Zhou and Y.-X. Zhao, Bull. Korean Chem. Soc., 2011, 32, 1403–1406 CrossRef CAS.
- C.-J. Li, J.-Z. Yang, S.-S. Yu, D.-M. Zhang, W. Xue, Y.-H. Yuan and N.-H. Chen, Zhongguo Tianran Yaowu, 2011, 9, 321–328 CAS.
- L. A. Tapondjou, L. B. T. Nyaa, P. Tane, M. Ricciutelli, L. Quassinti, M. Bramucci, G. Lupidi, B. K. Ponou and L. Barboni, Carbohydr. Res., 2011, 346, 2699–2704 CrossRef CAS.
- W.-W. Fu, J.-N. Fu, W.-M. Zhang, L.-X. Sun, Y.-H. Pei and P. Liu, Molecules, 2011, 16, 4371–4378 CrossRef CAS.
- Z. Zhang, X. Fang, Y.-H. Wang, G.-M. Liu, H. Xiao, X.-J. Hao and H.-P. He, J. Asian Nat. Prod. Res., 2011, 13, 838–844 CrossRef CAS.
- S. Sugimoto, K. Matsunami and H. Otsuka, Chem. Pharm. Bull., 2011, 59, 466–471 CrossRef CAS.
- N. B. Sarikahya, M. Pekmez, N. Arda, P. Kayce, N. U. K. Yavasoglu and S. Kirmizigul, Phytochem. Lett., 2011, 4, 415–420 CrossRef CAS.
- A. Sosa, C. Rosquete, L. Rojas, L. Pouysegu, S. Quideau, T. Paululat, A.-C. Mitaine-Offer and M.-A. Lacaille-Dubois, Helv. Chim. Acta, 2011, 94, 1850–1859 CrossRef CAS.
- H. Zhang, A. K. Samadi, K. V. Rao, M. S. Cohen and B. N. Timmermann, J. Nat. Prod., 2011, 74, 477–482 CrossRef CAS.
- Y. Ding, H.-F. Tang, J.-B. Wang, D. Liu, X.-R. Tian, X.-Y. Wang and X.-M. Zhou, Biochem. Syst. Ecol., 2011, 39, 236–239 CrossRef CAS.
- X.-Y. Wang, X.-L. Chen, H.-F. Tang, H. Gao, X.-R. Tian and P.-H. Zhang, Planta Med., 2011, 77, 1550–1554 CrossRef CAS.
- Q.-H. Wang, J. Zhang, X. Ma, X.-Y. Ye, B.-Y. Yang, Y.-G. Xia and H.-X. Kuang, Zhongguo Tianran Yaowu, 2011, 9, 17–21 CAS.
- G. Timite, A.-C. Mitaine-Offer, T. Miyamoto, C. Tanaka, J.-F. Mirjolet, O. Duchamp and M.-A. Lacaille-Dubois, Phytochemistry, 2011, 72, 503–507 CrossRef CAS.
- T. Morikawa, X. Li, E. Nishida, S. Nakamura, K. Ninomiya, H. Matsuda, M. Hamao, O. Muraoka, T. Hayakawa and M. Yoshikawa, Chem. Pharm. Bull., 2011, 59, 889–895 CrossRef CAS.
- K. Yang, Y. Li, L. Ge and Z. Qin, Adv. Mater. Res. (Durnten-Zurich, Switz.), 2011, 236–238, 1731–1737 CAS.
- M. Tene, P. Chabert, O. Note, T. J. N. Kenla, P. Tane and A. Lobstein, Phytochem. Lett., 2011, 4, 89–92 CrossRef CAS.
- J.-G. Luo, X. Chen and L.-Y. Kong, Chem. Pharm. Bull., 2011, 59, 518–521 CrossRef CAS.
- D. Du, L. Fang, J. Qu, S. Yu, S. Ma, H. Lv, J. Liu, Y. Liu, J. Wang and X. Wang, Planta Med., 2011, 77, 1631–1638 CrossRef CAS.
- S. Y. Lee, J. S. Kim, S. H. Shim and S. S. Kang, Bull. Korean Chem. Soc., 2011, 32, 3650–3654 CrossRef CAS.
- Q. Wen, D. Yuan, K.-H. Xie, T.-Z. Cai and H.-Z. Fu, J. Asian Nat. Prod. Res., 2011, 13, 869–878 CrossRef CAS.
- Q. Chen, J.-G. Luo and L.-Y. Kong, Carbohydr. Res., 2011, 346, 2206–2212 CrossRef CAS.
- N. A. Khan, Nat. Prod. Res., 2011, 25, 1687–1694 CrossRef CAS.
- A. Tava, L. Pecetti, M. Romani, M. Mella and P. Avato, J. Agric. Food Chem., 2011, 59, 6142–6149 CrossRef CAS.
- B. Hernandez-Carlos, A. Gonzalez-Coloma, A. U. Orozco-Valencia, M. V. Ramirez-Mares, M. F. Andres-Yeves and P. Joseph-Nathan, Phytochemistry, 2011, 72, 743–751 CrossRef CAS.
- Y.-X. Zhao, W.-J. Liang, H.-J. Fan, Q.-Y. Ma, W.-X. Tian, H.-F. Dai, H.-Z. Jiang, N. Li and X.-F. Ma, Carbohydr. Res., 2011, 346, 1302–1306 CrossRef CAS.
- W.-J. Liang, Q.-Y. Ma, H.-Z. Jiang, J. Zhou, J. Pang and Y.-X. Zhao, Chem. Nat. Compd., 2012, 47, 935–939 CrossRef CAS.
- X.-m. Song, Y. Liu and B.-c. Cai, Shenyang Yaoke Daxue Xuebao, 2010, 27, 627–629 CAS , 647.
- M. Z. Getiya, M. A. Gabelaya, V. D. Mshvildadze, A. Pichette, S. Lavoie and G. E. Dekanosidze, Chem. Nat. Compd., 2011, 47, 764–766 CrossRef CAS.
- A. I. Hamed, M. Masullo, M. G. Sheded, U. A. Mahalel, M. M. Tawfik, A. Perrone and S. Piacente, Phytochem. Lett., 2011, 4, 353–356 CAS.
- H.-X. Kuang, H.-W. Li, Q.-H. Wang, B.-Y. Yang, Z.-B. Wang and Y.-G. Xia, Molecules, 2011, 16, 4642–4651 CrossRef CAS.
- J.-S. Jiang, Z.-Z. Liu, Z.-M. Feng, Y.-N. Yang and P.-C. Zhang, J. Asian Nat. Prod. Res., 2011, 13, 276–280 CrossRef CAS.
- I. L. Acebey-Castellon, L. Voutquenne-Nazabadioko, D. T. M. Huong, N. Roseau, N. Bouthagane, D. Muhammad, M. D. E. Le, S. C. Gangloff, M. Litaudon, T. Sevenet, V. H. Nguyen and C. Lavaud, J. Nat. Prod., 2011, 74, 163–168 CrossRef CAS.
- R.-X. Deng, W.-L. Duan, P. Liu, Y.-L. Yang and W.-P. Yin, J. Asian Nat. Prod. Res., 2011, 13, 230–237 CrossRef CAS.
- V. T. T. Thanh, V. C. Pham, H. H. Nguyen, H. D. T. Mai, H. N. T. Minh, V. H. Nguyen, M. Litaudon, F. Gueritte and V. M. Chau, Eur. J. Org. Chem., 2011, 2011, 4108–4111 CrossRef CAS.
- M. A. Tantry, R. Khan, S. Akbar, A. R. Dar, A. S. Shawl and M. S. Alam, Chin. Chem. Lett., 2011, 22, 575–579 CrossRef CAS.
- Y.-P. Ma, N. Li, J. Gao, K.-L. Fu, Y. Qin, G.-Y. Li and J.-H. Wang, Helv. Chim. Acta, 2011, 94, 1881–1887 CrossRef CAS.
- Q.-X. Wu, Y.-B. Su and Y. Zhu, Fitoterapia, 2011, 82, 493–496 CrossRef CAS.
- P. Somwong, R. Suttisri and A. Buakeaw, Fitoterapia, 2011, 82, 1047–1051 CrossRef CAS.
- F. C. Silva, V. G. Rodrigues, L. P. Duarte, G. D. F. Silva, R. R. S. Miranda and S. A. V. Filho, J. Chem. Res., 2011, 35, 555–557 CrossRef CAS.
- F. C. Silva, L. P. Duarte, G. D. F. Silva, S. A. V. Filho, I. S. Lula, J. A. Takahashi and W. S. T. Sallum, J. Braz. Chem. Soc., 2011, 22, 943–949 CrossRef CAS.
- S. V. Fannang, V. Kuete, C. M. Djama, M. D. J. Dongfack, J. D. Wansi, F. Tillequin, E. Seguin, E. Chosson and J. Wandji, Chin. Chem. Lett., 2011, 22, 171–174 CrossRef CAS.
- G. Li, D. Wang and S. Xu, Nat. Prod. Res., 2011, 25, 136–140 CrossRef CAS.
- M. L. Kennedy, G. G. Llanos, S. Castanys, F. Gamarro, I. L. Bazzocchi and I. A. Jimenez, Chem. Biodiversity, 2011, 8, 2291–2298 CAS.
- G. Chen, H. Ren and C. Yu, Chem. Nat. Compd., 2011, 47, 918–920 CrossRef.
- S. Iqbal, A. Khan, V. U. Ahmad, M. A. Khan, S. Bader, U. Farooq, S. S. Khan, A. Zahoor and R. B. Tareen, Nat. Prod. Commun., 2011, 6, 179–182 CAS.
- W. Zhao, J. X. Pu, X. Du, Y. L. Wu, Y. Zhao, F. He, H. B. Zhang, Y. B. Xue, W. L. Xiao, G. Q. Chen and H. D. Sun, Arch. Pharmacal Res., 2011, 34, 2007–2014 CrossRef CAS.
- C. Wu, L. Wang, X.-X. Yang, Y.-H. Duan, Y. Dai, R.-W. Jiang, W.-C. Ye and Y.-L. Li, J. Asian Nat. Prod. Res., 2011, 13, 434–439 CrossRef CAS.
- A. Mittal and M. Ali, Int. Res. J. Pharm., 2011, 2(9), 52–54 CAS.
- S. Begum, S. Perwaiz, B. S. Siddiqui, S. Khan, S. Fayyaz and M. Ramzan, Chem. Biodiversity, 2011, 8, 850–861 CAS.
- B.-b. Zhang, K. Shi, Z.-x. Liao, Y. Dai and Z.-h. Zou, Fitoterapia, 2011, 82, 854–860 CrossRef CAS.
- S. Y. Lee, J. S. Kim, R. J. Choi, Y. S. Kim, J.-H. Lee and S. S. Kang, Chem. Pharm. Bull., 2011, 59, 742–746 CrossRef CAS.
- K. Mazumder, E. R. O. Siwu, S. Nozaki, Y. Watanabe, K. Tanaka and K. Fukase, Phytochem. Lett., 2011, 4, 287–291 CrossRef CAS.
- R. T. Kengap, G. D. W. F. Kapche, J.-P. Dzoyem, I. K. Simo, P. Ambassa, L. P. Sandjo, B. M. Abegaz and B. T. Ngadjui, Helv. Chim. Acta, 2011, 94, 2231–2238 CrossRef CAS.
- G. Topcu, G. Yapar, Z. Turkmen, A. C. Goren, S. Oksuz, J. K. Schilling and D. G. I. Kingston, Phytochem. Lett., 2011, 4, 421–425 CrossRef CAS.
- T. Sasaki, W. Li, H. Morimura, S. Li, Q. Li, Y. Asada and K. Koike, Chem. Pharm. Bull., 2011, 59, 1396–1399 CrossRef CAS.
- J. Hu, X.-Q. Chen and Q.-S. Zhao, J. Asian Nat. Prod. Res., 2011, 13, 105–110 CrossRef CAS.
- Y. Zhang, L.-J. Li, P. Zhang, H.-F. Pi, H.-L. Ruan and J.-Z. Wu, Helv. Chim. Acta, 2011, 94, 2207–2214 CrossRef CAS.
- L. P. Lin, W. Qu and J. Y. Liang, Chin. Chem. Lett., 2011, 22, 697–700 CrossRef CAS.
- X. N. Nguyen, B. H. Tai, T. H. Quang, P. V. Kiem, C. V. Minh, H. N. Nguyen, J.-H. Kim, L.-R. Im, Y.-M. Lee and Y. H. Kim, Bioorg. Med. Chem. Lett., 2011, 21, 1777–1781 CrossRef CAS.
- X.-Y. Xu, X.-H. Yang, S.-Z. Li and Q.-S. Song, J. Asian Nat. Prod. Res., 2011, 13, 1008–1013 CrossRef CAS.
- W.-J. Zuo, H.-F. Dai, J. Chen, H.-Q. Chen, Y.-X. Zhao, W.-L. Mei, X. Li and J.-H. Wang, Planta Med., 2011, 77, 1835–1840 CrossRef CAS.
- Y.-Y. Che, N. Li, L. Zhang and P.-F. Tu, Zhongguo Tianran Yaowu, 2011, 9, 22–25 CAS.
- W.-H. Cai, K. Matsunami, H. Otsuka and Y. Takeda, Am. J. Plant Sci., 2011, 2, 609–618 CrossRef CAS.
- E. Amin, S. S. El-Hawary, M. M. Fathy, R. Mohammed, Z. Ali, N. Tabanca, D. E. Wedge, J. J. Becnel and I. A. Khan, Planta Med., 2011, 77, 488–491 CrossRef CAS.
- C. L. Lencina, C. M. C. de, I. Zancanaro, G. Gosmann, V. S. Pires, P. Sonnet, D. Guillaume and E. P. Schenkel, Quim. Nova, 2011, 34, 222–225 CrossRef CAS.
- X. Cheng, J. Qin, Q. Zeng, S. Zhang, F. Zhang, S. Yan, H. Jin and W. Zhang, Planta Med., 2011, 77, 2061–2065 CrossRef CAS.
- G. Siedenburg and D. Jendrossek, Appl. Environ. Microbiol., 2011, 77, 3905–3915 CrossRef CAS.
- R. Badoni, D. K. Semwal, P. P. Badoni, S. K. Kothiyal and U. Rawat, Chin. Chem. Lett., 2011, 22, 81–84 CrossRef CAS.
- T. Řezanka, L. Siristova, K. Melzoch and K. Sigler, Lipids, 2011, 46, 249–261 CrossRef.
- M. Isaka, S. Palasarn, S. Supothina, S. Komwijit and J. J. Luangsa-ard, J. Nat. Prod., 2011, 74, 782–789 CrossRef CAS.
- J.-T. Fan, B. Kuang, G.-Z. Zeng, S.-M. Zhao, C.-J. Ji, Y.-M. Zhang and N.-H. Tan, J. Nat. Prod., 2011, 74, 2069–2080 CrossRef CAS.
- Z. Z. Ibraheim, A. S. Ahmed and Y. G. Gouda, Saudi Pharm. J., 2011, 19, 65–74 CrossRef CAS.
- R. S. T. Kamdem, P. Wafo, S. Yousuf, Z. Ali, A. Adhikari, S. Rasheed, I. A. Khan, B. T. Ngadjui, H.-K. Fun and M. I. Choudhary, Org. Lett., 2011, 13, 5492–5495 CrossRef CAS.
- C. Abbet, M. Neuburger, T. Wagner, M. Quitschau, M. Hamburger and O. Potterat, Org. Lett., 2011, 13, 1354–1357 CrossRef CAS.
- S.-H. Dong, C.-R. Zhang, L. Dong, Y. Wu and J.-M. Yue, J. Nat. Prod., 2011, 74, 1042–1048 CrossRef CAS.
- M. S. Gachet, O. Kunert, M. Kaiser, R. Brun, M. Zehl, W. Keller, R. A. Muñoz, R. Bauer and W. Schuehly, J. Nat. Prod., 2011, 74, 559–566 CrossRef CAS.
- N. Tanaka, R. Momose, A. Shibazaki, T. Gonoi, J. Fromont and J.-i. Kobayashi, Tetrahedron, 2011, 67, 6689–6696 CrossRef CAS.
- J.-H. Lee, K. H. Jang, Y.-J. Lee, H.-S. Lee, C. J. Sim, K.-B. Oh and J. Shin, J. Nat. Prod., 2011, 74, 2563–2570 CrossRef CAS.
- Y. Hasegawa, X. Gong and C. Kuroda, Nat. Prod. Commun., 2011, 6, 789–792 CAS.
| This journal is © The Royal Society of Chemistry 2013 |