Programming MOFs for water sorption: amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications†
Abstract
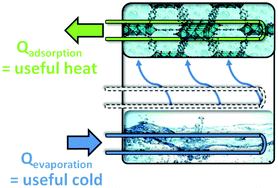
Sorption-based heat transformation and storage appliances are very promising for utilizing solar heat and waste heat in cooling or heating applications. The economic and ecological efficiency of sorption-based heat transformation depends on the availability of suitable hydrophilic and hydrothermally stable
- This article is part of the themed collection: Coordination Programming: Science of Molecular Superstructures Towards Chemical Devices

 Please wait while we load your content...
Please wait while we load your content...