Ethylenepolymerisation and oligomerisation with arene-substituted phenoxy-imine complexes of titanium: investigation of multi-mechanism catalytic behaviour†
Abstract
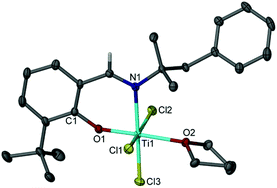
A range of unsubstituted (1,2) and 6-substituted (3–5) ortho-phenoxy-imine
- This article is part of the themed collection: Mechanistic Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...