A novel route of single step reactive calcination of copper salts far below their decomposition temperatures for synthesis of highly active catalysts†
Abstract
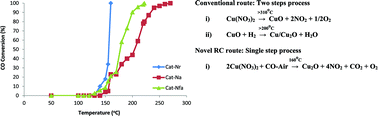
A novel route of reactive calcination (RC) of Cu-salts for the synthesis of highly active catalysts was studied for the first time. The route involved feeding a low concentration of a chemically reactive-CO–air mixture over the salts at low temperature (160 °C). The RC of the precursor produced copper species (Cu2O and CuO) in thermodynamic equilibrium, with the major nano-size Cu2O phase having a large surface area, while calcinations in stagnant air at 400 °C resulted in a CuO phase of larger crystallites with lower surface area. The optimum concentration of CO in the feed for RC was found to be 4.5%. The amazing activity of the novel catalysts over conventional ones in CO oxidation was associated with the presence of Cu2O and its unusual morphology as evidenced by N2 adsorption, XRD, XPS and FTIR characterization. The catalysts obtained by RC of various precursors showed activities for CO oxidation in the following order: acetate > hydroxide > oxalate > ammoniacal oxalate > nitrate between 130–160 °C while traditional route catalysts followed the same activity order but at higher temperatures (175–280 °C). Further, the activity order of the catalysts obtained by various calcination methods is as follows: RC > flowing-air > stagnant-air.

 Please wait while we load your content...
Please wait while we load your content...