Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dendritic architectures based on bis-MPA: functional polymeric scaffolds for application-driven research
Anna
Carlmark
,
Eva
Malmström
and
Michael
Malkoch
*
KTH Royal Institute of Technology, School of Chemical Science and Engineering, Fibre and Polymer Technology, Teknikringen 56-58, SE-100 44 Stockholm, Sweden. E-mail: Malkoch@kth.se
First published on 30th April 2013
Abstract
Dendritic polymers are highly branched, globular architectures with multiple representations of functional groups. These nanoscale organic frameworks continue to fascinate researchers worldwide and are today under intensive investigation in application-driven research. A large number of potential application areas have been suggested for dendritic polymers, including theranostics, biosensors, optics, adhesives and coatings. The transition from potential to real applications is strongly dictated by their commercial accessibility, scaffolding ability as well as biocompatibility. A dendritic family that fulfills these requirements is based on the 2,2-bismethylolpropionic acid (bis-MPA) monomer. This critical review is the first of its kind to cover most of the research activities generated on aliphatic polyester dendritic architectures based on bis-MPA. It is apparent that these scaffolds will continue to be in the forefront of cutting-edge research as their structural variations are endless including dendrons, dendrimers, hyperbranched polymers, dendritic-linear hybrids and their hybridization with inorganic surfaces.
 Anna Carlmark | Dr Anna Carlmark received her PhD in polymer technology in 2004 from Fibre and Polymer Technology at KTH Royal Institute of Technology in Sweden, under the supervision of Prof. Eva Malmström. Between 2004–2007 she was employed as a researcher in several industries in Sweden (GE Healthcare AB, Gyros AB and SweTree Technologies AB) before she rejoined the group of Prof. Malmström in 2007 as an associate professor in the division of Coating Technology at KTH. Her research focus is within the fields of controlled radical polymerization, the synthesis of complex macromolecular architectures, functional surfaces and (bio)fibre modifications. |
 Eva Malmström | Eva Malmström received her PhD in polymer technology in 1996 from KTH Royal Institute of Technology, Sweden. After a postdoctoral stay at IBM Almaden Research Center, US, she rejoined KTH as an assistant professor in 1997. In 2005 she became full professor and in 2009 she was appointed deputy president of KTH. She has published more than 100 papers and is the co-inventor of two patents and one of the founders of Polymer Factory Sweden AB. Professor Malmström's main research interests are the interplay between macromolecular architecture and properties, renewable materials and controlled polymerizations. |
 Michael Malkoch | Michael Malkoch is an Associate Professor in functional macromolecules at KTH Royal Institute of Technology, Sweden. He received a PhD in Polymer Technology from KTH in 2003 followed by a postdoctoral visit at Stanford University and the Materials Research Laboratory in Santa Barbara (UCSB). Currently, he holds two distinguish awards from the Swedish Council (VR) as a senior researcher in Biomedical engineering as well as a KAW Academy Fellow in Engineering Science. His research focuses on developing novel approaches for the construction of complex macromolecules that can be introduced in cutting edge fields of materials and biomedical sciences. |
1. Introduction
Historically, highly branched polymers were first mentioned by Flory in 1952 when he theoretically described the condensation of ABx-monomers to give polydisperse, statistically composed, highly branched architectures with a large number of end-groups.1 Interestingly, Flory assumed that this class of polymers would not be of significant interest, due to their poor material properties. Contradictory to Flory's assumption and as a complement to linear polymers, dendritic polymers with branched architectures and multiple representations of functional groups have emerged with a variety of structural variations and their use in potential cutting-edge applications is today widely assessed.2–4 It is apparent that current challenges in application-driven research are strongly dependent on the accessibility of complex and highly functional materials that can be tailored to an array of applications in a straightforward manner. This class of polymers was revisited by Vögtle et al.5 in 1978, describing the synthesis of branched polypropylene–amine structure followed by two parallel studies in 1985 from Tomalia et al.6 and Newkome et al.7 Currently, the family of dendritic polymers comprises a number of different architectures, including subclasses of monodisperse dendrons and dendrimers as well as polydisperse hyperbranched polymers and dendritic-linear hybrids. The intensive research on these scaffolds can be traced to the number of published scientific reports. As an illustrative example, a recent search, 8th of March 2013, on the keyword “dendrimers” on Web of Science, resulted in over 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 reports not including patents.
000 reports not including patents.
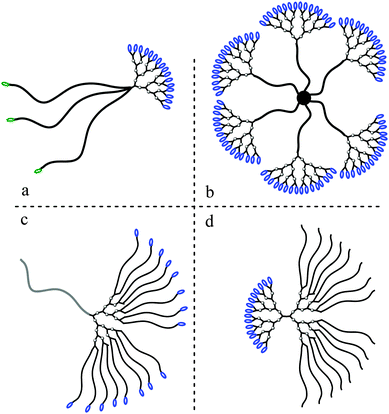
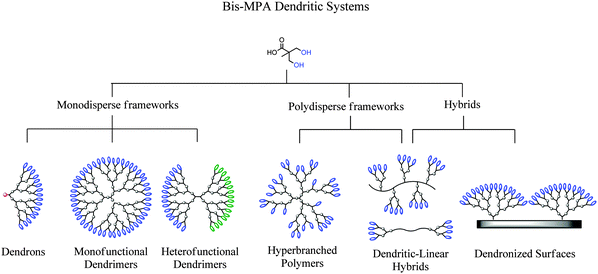
From the large number of reports on dendritic materials, a family that has been of particular interest is based on 2,2-bis(methylol)propionic acid (bis-MPA) as the building block. This simple aliphatic, pro-chiral, molecule with a molecular weight of 134.06 g mol−1 comprises two hydroxyls and one carboxylic group. It is readily available in bulk quantities and plays an important role in the synthesis of functional aliphatic polyesters. The bis-MPA molecule, being an AB2 monomer, has successfully been explored for the construction of a large number of dendritic materials, ranging from monodisperse dendrons and dendrimers to polydisperse hyperbranched polymers and dendritic-linear hybrids, Fig. 1. Furthermore, their hybridization with organic and inorganic surfaces has been accomplished to generate highly functional dendronized surfaces. Today, bis-MPA based dendritic polymers are commercially available, including hyperbranched BoltornTM as well as bis-MPA dendrimers and dendrons. The commercial availability of these highly functional polymers is a strong incitement on their potential use in both industry as well as academia for a wide array of applications, ranging from milligram to multi-ton scales. This critical review is the first of its kind detailing the overall research activities, in chronological order with respect to original reports, which have been generated on dendritic materials based on the bis-MPA monomer.
 | ||
| Fig. 1 Schematic overview of the sub-classes of dendritic polymers generated from bis-MPA. | ||
2. The chemistry of dendritic polymers based on bis-MPA
a. Hyperbranched polymers
Hyperbranched polymers (HBPs) have fascinated scientists and industrialists as a “poor man's alternative to dendrimers”. In contrast to the tedious and demanding synthetic protocols that are required to accomplish flawless and monodisperse dendrimers, the synthesis of hyperbranched polymers is often based on one-pot reactions, requiring essentially no work-up. The properties of hyperbranched polymers are similar to those of dendrimers; although polydisperse, their highly branched structure do not generally form chain entanglements and the branching also results in high solubility and low melt- and solution viscosity. The ease by which hyperbranched polymers are synthesized and their intrinsically different properties compared to linear polymers have spurred significant interest in HBPs over the last few decades and there are several excellent review chapters and books summarizing the area.8–10The first report describing the use of bis-MPA as an AB2-monomer for the construction of a dendritic macromolecule was published by Hult et al. in 1993.11 In this study, bis-MPA based allyl-functional oligoesters were used in coating applications. A modification of the synthetic strategy was later described, where bis-MPA was copolymerized with trimethylolpropane (TMP) in an acid-catalyzed condensation reaction to provide more control of the molecular weight and polydispersity.12 The reaction was carried out as a pseudo one-step reaction, by successive addition of monomers, in one-pot to increase the probability of building TMP-cored hyperbranched polyesters. In the first step, one equiv. of TMP and three equiv. of bis-MPA, corresponding to the stoichiometric ratio in a first generation dendrimer [G1], were reacted at 140 °C. However, since the outcome of the condensation reaction is based on statistics, a range of isomers and species having different molecular weights will form as the reaction progresses. After approximately 3 hours under reduced pressure, another portion of bis-MPA, corresponding to the theoretical amount required for the [G2] dendrimer, was added together with another catalytic portion of the acid and could be repeated several times to reach the desired bis-MPA to core ratio. The pseudo-one pot procedure was utilized to favor chain growth on the already formed oligoester backbone, rather than forming new oligoester skeletons. The copolymerization of bis-MPA with the polyol core (or chain stopper) is important to reduce the polydispersity of the final polymer, and to improve the molecular weight control, and also, to some extent, reduce the risk of unwanted side-reactions, such as cyclizations and etherifications. The straightforward synthetic procedure is still valid, and can be utilized for various suitable core moieties and end-groups and their combinations. Although hyperbranched polymers are simple to construct, challenging characterizations comes with the price due to their complex polydisperse nature.
These HBPs are often described in the literature by their trade name Boltorn™ and are globally provided by Perstorp AB, making them one of the mostly utilized HBPs in the literature. The original Boltorn™s are comprised of hydroxyl functionalities at the periphery and are named H20, H30 and H40, indicating that the stoichiometric ratio between the core and bis-MPA corresponds to the generation 2 [G2], 3 [G3] and 4 [G4] dendrimer accordingly. Scheme 1 conceptually depicts Boltorn H30. Other Boltorn derivatives include U3000, W3000, H2003, H2004, P1000, etc. The vast interest in the synthesis of hyperbranched polymers has fuelled the development of new characterization protocols that allow for the understanding of the structure–property interplay as summarized in a comprehensive review by Zagar and Zigon.13
![Idealized structure of one isomer of a pseudo-third generation [G3] hyperbranched polyester based on bis-MPA and PP50. The synthesis is conducted using 3 addition of bis-MPA, each corresponding to the theoretical amount of the corresponding dendrimer.](/image/article/2013/CS/c3cs60101c/c3cs60101c-s1.gif) | ||
| Scheme 1 Idealized structure of one isomer of a pseudo-third generation [G3] hyperbranched polyester based on bis-MPA and PP50. The synthesis is conducted using 3 addition of bis-MPA, each corresponding to the theoretical amount of the corresponding dendrimer. | ||
b. Dendrimers
The monodisperse nature of dendrimers typically requires robust chemical reactions coupled with tedious workup procedures. Consequently, most of the research activities have been limited to a few dendrimer scaffolds provided by a selected number of world-wide commercial sources. With their current commercial availability, dendrimers based on the bis-MPA monomer are today one of the most utilized dendritic platforms. While bis-MPA is the most commonly used abbreviation, alternative widely-used acronyms are bisMPA, BMPA, bis-HMPA dendrimers as well as PBisMPA. Since the initial synthetic report by Hult et al. in 1996,14 bis-MPA dendrimers have been constructed up to [G6], with a myriad of bis-MPA based dendritic structures published, including both traditional as well as heterofunctional dendrimers (HFDs). The introduction of the “click” concept by K. Barry Sharpless has provided a facile synthetic tool for the construction of dendrimers,15,16 which is today routinely used for the synthesis of sophisticated bis-MPA dendrimers.17,18 The most critical synthetic reports are described below and briefly summarized in Table 1.| Dendrimer | Strategy | Building blocks | Reaction | Steps | Generation | End groups | Total yield (%) | Year |
|---|---|---|---|---|---|---|---|---|
| Homofunctional | ||||||||

|
Convergent |

|
Esterification | 10 | [G4] |

|
15 | 199614 |
| Double exponential |

|
Esterification | 7 | [G4] |

|
56 | 199819 | |
| Divergent |

|
Esterification | 8 | [G6] |

|
48 | 200120 | |
| Divergent |

|
Esterification | 8 | [G4] |

|
47 | 200221 | |

|
Orthogonal |

|
Esterification/CuAAC | 4 | [G4] |

|
69 | 200725 |
| Orthogonal |

|
TEC/esterification | 5 | [G5] |

|
12 | 201027 | |
| Orthogonal |

|
TEC/CuAAC | 6 | [G6] |

|
8 | 201028 | |
|
Orthogonal |

|
TEC/esterification | 4 | [G4] |

|
26 | 201027 |
| Orthogonal |

|
Esterification/TEC | 5 | [G5] |

|
19 | 201027 | |
| Orthogonal |

|
CuAAC/DA | 3 | [G3] |

|
27 | 201029 | |
| Heterofunctional | ||||||||

|
Divergent |

|
Esterification | 12 | [G3–G3] |

|
39 | 200231 |
| Divergent/convergent |

|
Esterification/CuAAC | 12 | [G4–G4] |

|
57 | 200532 | |
| Divergent/convergent |

|
Esterification/SPAAC | 14 | [G3–G2] |

|
55 | 201133 | |
| Divergent/convergent |

|
Esterification/TEC | 12 | [G3–G3] |

|
20 | 201122 | |

|
Divergent |
|
Esterification | 6 | [G2] |

|
46 | 200735 |
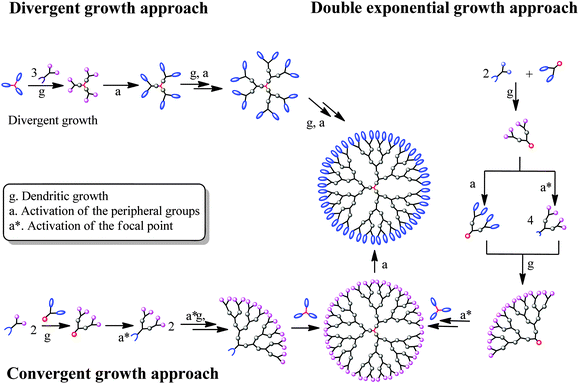 | ||
| Fig. 2 Overall strategies that have successfully been employed for the construction of homofunctional traditional bis-MPA dendrimers. | ||
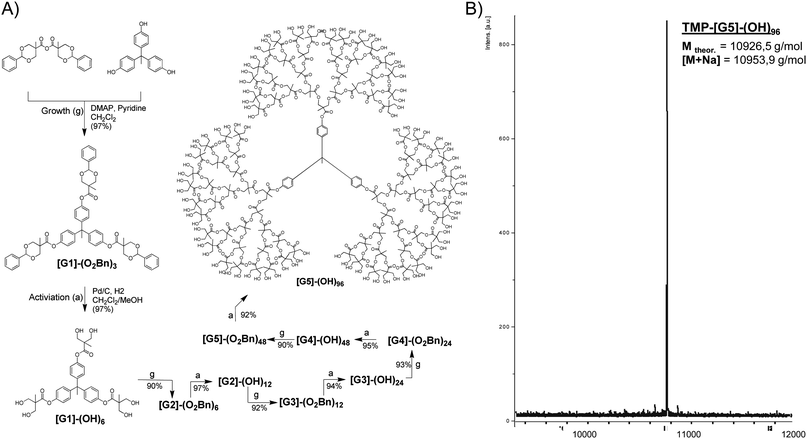 | ||
| Scheme 2 (A) Divergent growth approach for the construction of fully activated bis-MPA dendrimers utilizing an anhydride activated bis-MPA monomer. (B) Structural purity of a fifth generation bis-MPA dendrimers with 96 peripheral hydroxyl groups as shown by MALDI-TOF. | ||
Most recently, Malkoch et al. reported on the synthesis of traditional bis-MPA dendrimers that were divergently grown and contained a cleavable disulfide core. Upon reaching the desired generation, the dendrimers were selectively cleaved by dithiothreitol (DDT) in high yields generating dendritic macrothiols that were further reacted via UV initiated thiol–ene coupling (TEC) chemistry to result in sophisticated dendritic materials, in both organic as well as aqueous solutions.22 Today, all traditional bis-MPA dendrimers are commonly synthesized and accurately characterized via the divergent growth approach. The structural integrity of all monodisperse dendrimers is routinely analyzed by both NMR and MALDI-TOF MS. A typical MALDI spectrum displaying a single peak corresponding to a [G5] bis-MPA dendrimer is shown in Scheme 2B.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Scheme 3c.28 Each reaction reached completion within 1 h and the fully activated dendrimers, having either azide or alkene peripheral groups, were isolated through simple purification procedures. Interestingly, the 7.0 equiv. excess of thiol to ene required to reach a fully substituted [G5] dendrimer was found (by SEC) to generate 10% fraction of dendrimer–dendrimer intermolecular cross-coupled.
000 g mol−1, Scheme 3c.28 Each reaction reached completion within 1 h and the fully activated dendrimers, having either azide or alkene peripheral groups, were isolated through simple purification procedures. Interestingly, the 7.0 equiv. excess of thiol to ene required to reach a fully substituted [G5] dendrimer was found (by SEC) to generate 10% fraction of dendrimer–dendrimer intermolecular cross-coupled.
 | ||
| Scheme 3 Different accelerated and orthogonal growth strategies to homofunctional bis-MPA dendrimers employing (a) esterification and CuAAC; (b) TEC and esterification reactions; (c) TEC and CuAAC and (d) CuAAC and DA. | ||
Based on the modular nature to construct bis-MPA dendrimer scaffolds utilizing benign and accelerated reaction approaches, hybridization strategies with complementary monomers have also been reported. For instance, Kakkar et al. reported the successful construction of a fully clicked [G3] dendrimer utilizing CuAAC and DA reactions based on bis-MPA (AB2) and aromatic (CD2) monomers, Scheme 3d.29 The obtained dendrimers exhibited thermoresponsivity in which the dendritic scaffold disassembled via a retro-DA at physiologically relevant pH. As can be seen in Table 1, other bis-MPA dendrimer hybrids have been accomplished via orthogonal growth approaches with both thioglycerol as well as trimethylol propane as paired monomers.27 Most recently, hybridization via accelerated approaches combined both esterification and Michael addition reactions which resulted in a [G5] dendrimer with 128 hydroxyl peripheral groups.30
The first original paper on the construction of heterofunctional bis-MPA dendrimers was reported by Gillies and Fréchet in 2002.31 By combining the anhydride activated benzylidene and the acetonide protected bis-MPA monomers, a [G3] didendron based HFD was synthesized via esterification reactions in 12 consecutive steps employing both the convergent and divergent growth approaches, Fig. 3a. The HFD based on two dendrons was isolated in an overall yield of 39% and displayed 4 acetonide and 8 hydroxyl end groups. An alternative synthetic strategy was proposed by Hawker et al. in 2005 utilizing the chemoselectivity of the CuAAC reaction. Two separate dendrons were grown divergently to [G3] comprising in one end a single azide and eight hydroxyl groups and in the other end a primary acetylene in the focal point with 4 acetonide protective groups. The two dendrons were efficiently coupled in THF and isolated in 92% yield, Fig. 3b.32 Following a similar synthetic methodology as for the CuAAC strategy, both the UV initiated TEC as well as the strain-promoted azide–alkyne cycloaddition (SPAAC) have successfully been explored as alternate click reactions for the synthesis of HFDs based on didendrons with dual functionalities, Fig. 3c and d.22,33 The potential use of these scaffolds as in vivo drug delivery vehicles34 makes the TEC and SPAAC strategies of importance as they exclude the use of potentially toxic copper.
![Third generation [G3] HFD scaffolds based on bis-MPA and with exterior block dual functionalities, (a) HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide)4; (b) HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide)4, HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide); (c) HFD(e)-G3-(acetylene)8-b-G2-(acetylene-TMS)4 based on the nomenclature proposed by Walter and Malkoch for HFDs.17](/image/article/2013/CS/c3cs60101c/c3cs60101c-f3.gif) | ||
| Fig. 3 Third generation [G3] HFD scaffolds based on bis-MPA and with exterior block dual functionalities, (a) HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide)4; (b) HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide)4, HFD(e)-G3-(hydroxyl)8-b-G3-(acetonide); (c) HFD(e)-G3-(acetylene)8-b-G2-(acetylene-TMS)4 based on the nomenclature proposed by Walter and Malkoch for HFDs.17 | ||
Though HFDs based on didendron blocks have efficiently been constructed and characterized, their major drawback is coupled to the large number of required iterative reaction steps. For all the third generation dendrimers shown in Fig. 3, a minimum number of 10 reaction steps are required, not including monomer synthesis or further functionalization. An apparent consequence of the number of reaction steps limits their accessibility for the larger scientific audience. To simplify the synthesis, HFDs that display alternating peripheral dual functionality have been suggested as alternative scaffolds. By employing the divergent growth approach, a [G2] bis-MPA dendrimer was synthesized expressing eight cyclic carbonates, Scheme 4. A number of different amine functional substituents were selectively reacted via ring opening of the cyclic carbonates, resulting in HFDs in only four reaction steps and with 16 alternating heterofunctionalities at the periphery.35
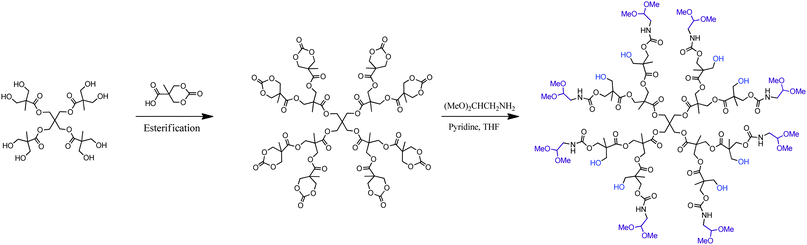 | ||
| Scheme 4 A simplified strategy for the construction of bis-MPA HFD(e)-G2-(hydroxyl)8-a-(acetal)8 with externally (e) alternating (a) dual functionality as proposed by Fréchet et al.35 | ||
c. Dendritic-linear polymer hybrids
Dendritic-linear polymer hybrids are sophisticated polymer structures containing linear chains as well as dendritic segments. These structures have been investigated since the first reports by Fréchet et al. in the early 1990's36–38 and since then, several fascinating structures have been produced over the years. Their synthesis require chemists skilled in both organic and polymer chemistry. The linear and dendritic segments can be combined in several different manners, resulting in an array of functional structures, many of which can be found in a review by Gitsov.39 An important aspect of their synthesis, compared to dendrimers, is the purification protocols employed which typically require simple precipitations in poor solvent. Due to the unique rheological, solution and solid-state properties of these polymers, it is hypothesized that they could find applications as nanometer-sized reactors, nanoporous sized thin-films, nanosponges and for non-covalent and site-specific modifications of glycoproteins, etc.39One great advantage of using the bis-MPA building block to produce hybrid structures is the intrinsic availability of hydroxyl end-groups, which can easily be functionalized in a benign fashion by a number of chemical reactions. Furthermore, the bis-MPA dendritic structures can be divergently grown from any type of hydroxyl or amine group. Below follows a summary of bis-MPA based dendritic-linear polymer hybrids reported in the literature.
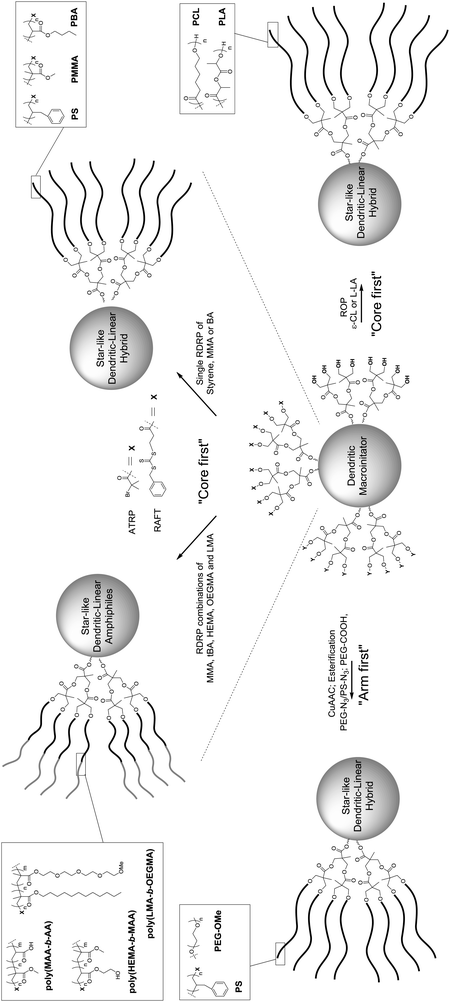 | ||
| Scheme 5 Overall strategies described in the literature for the construction of star-like dendritic-linear hybrids based on bis-MPA. | ||
Hedrick et al. reported the first ROP of a bis-MPA-based core molecule, polymerizing ε-CL from [G1] and [G2] dendritic macroinitiators.40 In an extension of this work, both L-LA and ε-CL were polymerized at 110 °C from G1–G4 bis-MPA dendrimers utilizing a catalytic amount of Sn(Oct)2, Scheme 5.41,42 Hence, in the case of the G4 dendrimer, 48 linear chains were grown from the core molecule with a resulting molecular weight as high as 115 kDa and polydispersities as low as 1.17.42 The resulting hybrid structures were semicrystalline and formed films. Measurements of the relationship between hydrodynamic volume vs. molecular weight showed a deviation from linearity, typically seen in dendrimers of higher generation. Hult et al. further expanded this methodology to include HBP Boltorn H30, a [G3] bis-MPA dendrimer and a dendron as macroinitiators for ROP of ε-CL.43 In this study it could be concluded that with lower Mw of the grafted PCL, unreacted OH-groups of the macroinitiators remained. The hybrid structures were compared to the linear PCL and the rheological properties were evaluated, showing that the branched structures had a considerably lower zero shear rate viscosity (η0) than the linear PCL of the same molecular weight.
Bis-MPA scaffolds have also been utilized as macroinitiators in RDRP. In the first reports, Hedrick and Miller et al. synthesized [G1] and [G2] dendrimer macroinitiators where the hydroxyl groups were converted to tert-Br groups to be exploited as initiating sites for Atom Transfer Radical Polymerization (ATRP) of methyl methacrylate (MMA)44 and for block-copolymer amphiphiles composed of poly(MMA-b-AA).45 In an alternative approach, two ATRP initiating groups were first attached to the bis-MPA monomer followed by the attachment to [G1] and [G2] dendrimers and dendrons.46 ATRP of MMA was successfully performed from all initiating sites except for the [G2] dendrimer having an average of 10–12 polymer chains of 12 possible initiating sites. Similarly, Grayson et al. utilized first and second generation bis-MPA dendrimers with six and 12 reactive sites for ATRP of oligo (ethylene glycol) methacrylate (OEGMA) and lauryl methacrylate (LMA). The resulting star-like amphiphilic block-copolymers were evaluated as transdermal carriers.47 Reversible-Addition Fragmentation Chain Transfer (RAFT) polymerizations have also been explored in a similar manner by Barner-Kowollik et al. The peripheral hydroxyl groups of lower generation bis-MPA dendrons48 as well as hyperbranched Boltorn H2049 were converted into RAFT agents by a reaction with 3-benzylsulfanylthiocarbonylsulfanylpropionic acid. The macroinitiators were utilized for RAFT polymerization of butyl acrylate (BA) and styrene (S).
The “arm-first” approach has also been successfully investigated for the construction of star like dendritic-linear hybrids. A direct esterification coupling between carboxylic acid monofunctional PEG of 5 kDa or 10 kDa in molecular weight and Boltorn H30 and H40 was successfully reported by Nyström et al.50 Anti-cancer agent doxorubicin (DOX) was entrapped within the hybrids and the structures were evaluated in terms of morphology, particle size, drug encapsulation efficiency, in vitro release, cellular uptake, cytotoxicity and apoptosis induction. Boltorn H30 with PEG10k chains was found to be the most promising in terms of controlled release and stability. A similar esterification methodology was further adapted by Shen et al. and resulted in a [G5] orthogonally grown bis-MPA dendrimer with 56 peripheral 2 kDa PEG chains.30 The “arm-first” strategy has also been exploited via click chemistry. Grayson et al. decorated first and second generation bis-MPA dendrimers with primary acetylenes that were subsequently reacted via CuAAC reactions with azido functional PS and PEG. These star-structures were carefully investigated using MALDI-TOF, revealing the formation of “perfect” stars.51
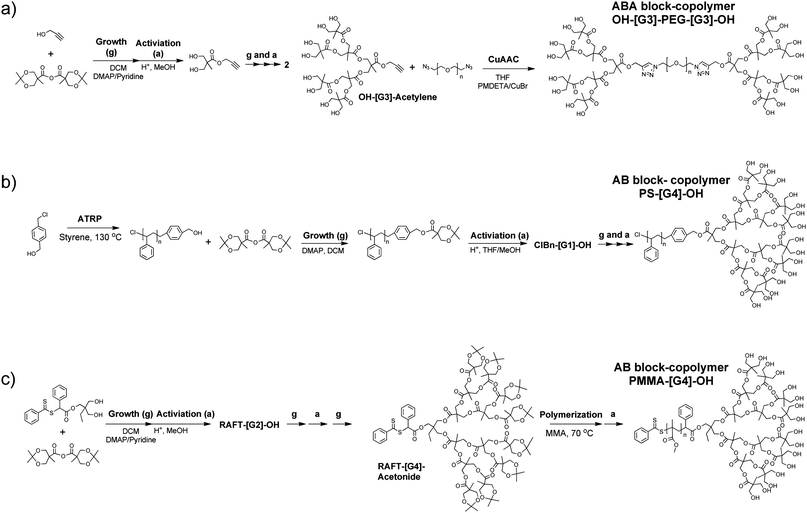 | ||
| Scheme 6 Different approaches to generate dendritic-linear hybrids as AB, ABA and ABC block-copolymers. | ||
Dendritic-linear hybrids based on dendritic bis-MPA structures which do not fall within the categories mentioned above have also been reported. For example, Hedrick et al. produced dendritic-linear AB, A2B, AB2, A2B2 and A3B3 hybrid structures, where the A-block is a bis-MPA dendron of [G1] to [G3] and the B-block is a linear polymer chain, for example PCL.57 The macroinitiators, i.e. the OH-functional dendrons, were orthogonally protected, meaning that ROP57 and ATRP58 could be performed from selected end-groups of the dendron structure, leaving a set number of the OH-end groups un-grafted. A schematic representation of an AB3 with dual-functionality can be seen in Fig. 4a. Hawker and Qiao described the construction of a core cross-linked star polymer in which an AB block-copolymer, tert-Br-PS-[G4]-acetonide, was crosslinked at the Br-chain site by copolymerization with divinyl benzene, Fig. 4b.59 ABCx hybrids were recently produced by Malkoch et al., using either CuAAC or thiol–ene chemistry.60 In their approach, bis-MPA dendrons, having an alkyne group or an ene-group in the focal point, were synthesized up to the fourth generation. The peripheral OH-groups were used as macroinitiators for ROP of ε-CL and subsequently PEG chains, either azide or thiol functionalized, were coupled to the focal point of the dendron. Hence, ABCx amphiphilic structures were produced, having a single PEG chain coupled to a dendron having 2, 4, 8, or 16 PCL chains attached to it. In Fig. 4c, a schematic representation of ABC16 hybrids based on a [G4] dendron with 16 PCL chains is shown. Fréchet et al. have coined the term “bow-ties” for a class of dendritic hybrid structures, which are based on heterofunctional dendrimers (HFDs).31,61 The structure consists of two, covalently attached, dendritic segments, which may be of different generation, and linear chains are attached to one of the dendritic segments, examples are shown in Fig. 4d. Structures based on dendritic segments of bis-MPA and linear segments of PEG have been synthesized, using both convergent and divergent methods and the generation of the dendritic segments was varied as well as the molecular weight of the PEG chains.31
d. Dendronized polymer hybrids
Dendronized polymers can be considered a subgroup of dendritic-linear polymer hybrids, as they are a form of comb-polymers composed of a linear backbone onto which dendrons have been attached to each repeating unit.62 However, since these macromolecular hybrids predominantly consist of dendritic components they are often considered as an isolated subgroup of dendritic polymers. The field of dendronized polymers has been extensively studied and reviewed by Schlüter et al.63–65 After the first, published, reports of these materials,66,67 they have attracted great interest due to their rod-like and cylindrical shapes and their potential to be used as catalyst supports, energy transfer, light harvesting, and/or electrically conducting materials, nanoobjects for surface patterning, gene-delivery, etc.65,68Dendronized polymers based on bis-MPA have been produced both through the ‘grafting-to’69 and the ‘macromonomer’ methodology.70
![(I) Grafting-to via divergent growth utilizing iterative esterification/deprotection reactions71 and (II) grafting-to through convergent coupling of a third generation [G3] macrothiol dendron to allyloxy poly(HEMA) exploiting the UV initiated TEC click chemistry.22 (III) ATRP polymerization via the macromonomer approach of a second generation [G2] bis-MPA dendron with a flexible spacer to the polymerizable group.73](/image/article/2013/CS/c3cs60101c/c3cs60101c-s7.gif) | ||
| Scheme 7 (I) Grafting-to via divergent growth utilizing iterative esterification/deprotection reactions71 and (II) grafting-to through convergent coupling of a third generation [G3] macrothiol dendron to allyloxy poly(HEMA) exploiting the UV initiated TEC click chemistry.22 (III) ATRP polymerization via the macromonomer approach of a second generation [G2] bis-MPA dendron with a flexible spacer to the polymerizable group.73 | ||
A critical drawback of the convergent methodology is the employment of large excess dendron precursors that are onerous to construct. Nonetheless, the convergent approach in which bis-MPA dendrons are efficiently coupled to linear polymers has been described briefly in the literature. Malmström et al. utilized a cellulose derivative, hydroxypropyl cellulose (HPC), as the backbone to which bis-MPA dendrons were convergently coupled, up to [G3].74 The size of the dendronized polymers was studied in solid states by spin-casting on mica and graphite surfaces followed by investigation of the surfaces with tapping-mode AFM. The polymers adopted a spherical shape and their diameters were estimated to be ca. 30–70 nm. In another synthetic route, Malkoch et al. utilized the UV initiated TEC click chemistry to successfully couple “macrothiol” to fully allylated poly(HEMA). All allyls were consumed within 1.5 h of UV exposure, resulting in a [G3] dendronized polymer with a theoretical molecular weight of about 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Scheme 7(II). Dendronized polymers that display the hybridization between bis-MPA and Fréchet-type arylether dendrons have also been successfully described in the literature. Bis-MPA dendrons were divergently grown from poly(p-hydroxylstyrene) followed by an activation step with primary acetylene to which the [G3] arylether dendron with a single azido group was convergently clicked together resulting in a [G6] dendronized polymer with a detected molecular weight of 7020 kDa.75 More recently, Grayson et al. exploited CuAAC reactions for the development of a new class of cyclic dendronized polymers which were successfully constructed from both octanol deep-cavity cavitand76,77 as well as cyclic poly(p-hydroxystyrene).78
000 g mol−1, Scheme 7(II). Dendronized polymers that display the hybridization between bis-MPA and Fréchet-type arylether dendrons have also been successfully described in the literature. Bis-MPA dendrons were divergently grown from poly(p-hydroxylstyrene) followed by an activation step with primary acetylene to which the [G3] arylether dendron with a single azido group was convergently clicked together resulting in a [G6] dendronized polymer with a detected molecular weight of 7020 kDa.75 More recently, Grayson et al. exploited CuAAC reactions for the development of a new class of cyclic dendronized polymers which were successfully constructed from both octanol deep-cavity cavitand76,77 as well as cyclic poly(p-hydroxystyrene).78
In 2004, Malmström et al. reported the first dendronized polymers based on bis-MPA through the ‘macromonomer’ route.73 In this case, Scheme 7(III), dendronized bis-MPA monomers of [G1] to [G3] with an acrylic polymerizable group separated by a flexible 10 C spacer were polymerized by ATRP.79 All three macromonomers followed first-order kinetics and were polymerized to high conversions. Following the same synthetic concept, Hult et al. produced dendronized polymers where a G1 macromonomer was synthesized by reaction of HEMA with bis-MPA, which was subsequently polymerized by ATRP. From the resulting G1 dendronized polymer, now only having a 2 C spacer in between the polymer backbone and the dendron side-chain, dendrons were divergently grown up to [G4].80–82 The bulk82 and solution80 properties of the dendronized polymers were carefully studied, where 1H NMR self-diffusion and longitudinal relaxation data showed that the polymers had an elongated rod-like structure in solution and that the higher generation dendronized polymers had a larger rod diameter, which approximately doubled when the generation was increased from two to four. The same group synthesized macromonomers from 5-norbornene-2-methanol, to which bis-MPA was divergently grown from [G1] to [G4].83 The resulting dendronized norbornene macromonomers were subsequently polymerized by ROMP, employing Grubb's first or second generation catalyst. The first generation catalyst resulted in polymers having fairly controlled dispersity (ranging between 1.2 and 3.7) and molecular weights of the dendronized polymers obtained from the [G4] macromonomer were in the range of 120 kDa. Similarly, Choi et al. synthesized a bis-MPA based macromonomer having a norbornene unit in the structure, although a biphenyl group was incorporated as a rigid spacer in between the polymerizable norbornene unit and the dendron.84 Macromonomers up to [G5] were successfully polymerized by ROMP, utilizing Grubb's third generation catalyst. The resulting polymers had low dispersities, between 1.04 and 1.51, and molecular weights as high as 1036 kDa were reported for the [G5] macromonomer. Furthermore, the rod-like conformation was confirmed and visualized as individual polymer chains by AFM.
Most recently, Malkoch and Barner-Kowollik et al. investigated the combination of dendrons and high temperature acrylate polymerization to obtain dendronized polymers.85 Bis-MPA-based dendronized acrylate macromonomers ranging from [G1] to [G3] were synthesized by CuAAC, incorporating a flexible 6 or 9 carbon spacer in the structure. The acrylate monomers were then polymerized by auto-initiated high temperature polymerization resulting in vinyl terminated oligomers, where the degree of polymerization decreased with increasing generation.
f. Dendronized surfaces based on bis-MPA
The immobilization of dendritic structures on solid surfaces creates dendronized surfaces, sometimes denoted forests. This topic has been thoroughly investigated as dendronized surfaces can amplify functional groups and surface area, resulting in highly controlled nanostructures.68 The dendritic structures can be immobilized either via physiosorption or by covalent attachment. In physiosorption the dendritic structure normally self-assembles onto the surface, forming either monolayers or multilayers. In the covalent attachment approach, the dendritic structures, typically a dendron, can be chemically bonded to the surface through the focal point or by the peripheral groups. Furthermore, the covalent attachment of dendrons to surfaces can be performed through both ‘graft-to’ and the ‘grafting-from’ approaches. In the ‘graft-to’ route, preformed dendrons or dendrimers are convergently, chemically bound to the surface, and in the ‘grafting-from’ method, the dendrons are grown from the surface. This can occur either similar to dendritic growth, where generations are divergent grown from the surface, or by forming a hyperbranched polymer directly on the surface through, for example, self-condensing vinyl polymerization or normal condensation polymerization.86 Considering the facile preparation of bis-MPA based dendritic structures with any hydroxyl functionalities there are surprisingly few reports in the literature detailing their attachment to solid surfaces.![(A) Sixth generation [G6] dual functional dendronized cellulose surface by a combination of grafting to and grafting from approach. (B) Grafting from methodology for the fabrication of fourth generation [G4] dendronized Au surfaces.](/image/article/2013/CS/c3cs60101c/c3cs60101c-s8.gif) | ||
| Scheme 8 (A) Sixth generation [G6] dual functional dendronized cellulose surface by a combination of grafting to and grafting from approach. (B) Grafting from methodology for the fabrication of fourth generation [G4] dendronized Au surfaces. | ||
3. Foreseen applications of dendritic materials based on the bis-MPA platform
From the number of available scientific papers it is apparent that dendritic materials based on bis-MPA have potential in an array of applications. Their facile synthesis, postfunctionalization and hybridization are a continuous topic towards application driven research. Currently, a large number of carefully selected substituents, not including polymeric hybrids, have successfully been attached, both as a core as well as peripheral groups, Fig. 5, resulting in advanced dendritic frameworks for the evaluation in solution, networks as well as thin film applications.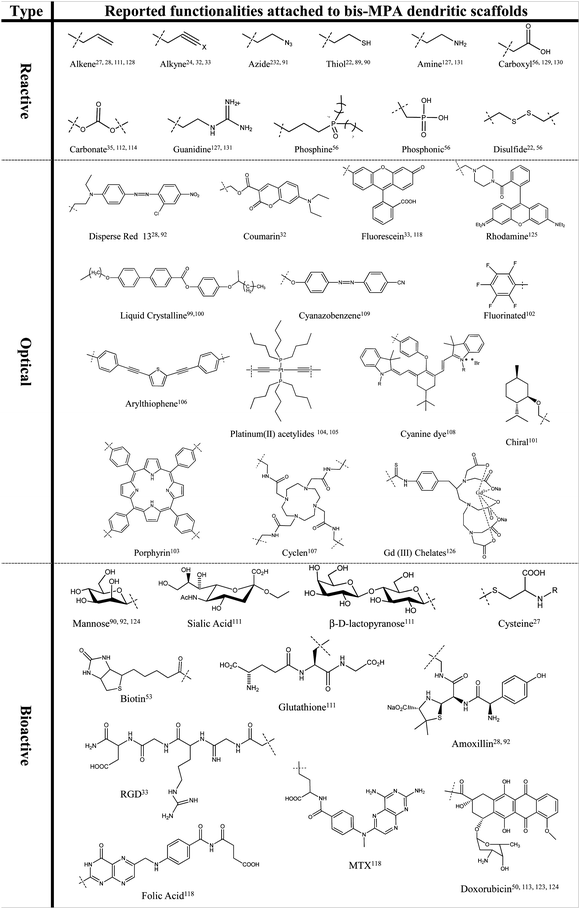 | ||
| Fig. 5 Selection of substituents attached to bis-MPA architectures. | ||
a. Optical
The branched and layered architecture of bis-MPA dendrimers displaying multiple reactive hydroxyl groups at the periphery has enabled their exploitation as functional probes in optical applications. Ferroelectric liquid coralline dendrimers up to [G3] with 24 mesogene containing groups were successfully synthesized revealing a variety of mesophases including the smectic C* phase as well as ferroelectricity.99,100 Peripheral chiral101 as well as fluorinated102 bis-MPA dendrimers have been synthesized providing site isolation of divalent and trivalent lanthanides in which their luminescence efficiency was evaluated. Metal-ion containing macrocycles have also been introduced as core moieties of bis-MPA dendrimers. Optically active porphyrin was successfully decorated with a dendritic shell via the divergent growth approach.103 Nonlinear optical (NLO) chromophore platinum(II) acetylides were impeded in a bis-MPA dendritic shell and optical-power limiting (OPL) measurement showed improved OPL properties with increased generation.104,105 Arylthiophene cored bis-MPA dendrimers were straightforwardly synthesized via the divergent growth strategy to [G4] and their optical-power limiting (OPL) properties were observed at 532 nm, Fig. 6a.106 The emergence of the click concept was also reported for the convergent coupling of the [G4] dendron to cyclen.107 An increase in luminescence decay time was detected for higher generation dendrimers with incorporated lanthanide metal ion Europium. Furthermore, the stable triazole linkage generated from the click reaction was found to act as a sensitizer. Employing a similar strategy, [G2] and [G3] bis-MPA dendrons were efficiently coupled to an azide functional cyanine dye.108 Photophysical characterization elucidated an intense absorption and emission in the near-infrared. Linear-dendritic block-copolymers comprising a single PEG chain (Mw 1, 2 and 5 kDa) coupled to [G1] to [G4] bis-MPA dendrons were decorated with mesogenic and photochromic cyanazobenzene units and the aggregation behavior of the amphiphilic hybrid structures was studied in water solution.109 In this case, the hybrids were found to form cylindrical micelles, sheet-like micelles, tubular micelles as well as polymer vehicles, depending on the generation of dendrons and Mw of the PEG chain.![(a) Arylthiophene cored [G4] bis-MPA dendrimers for optical-power limiting (OPL) applications,106 (b) ordered honeycomb membranes from core crosslinked star AB block-copolymer hybrids consisting of PS and bis-MPA dendrons55 and (c) ultrathin films via LbL methodology from monodisperse bis-MPA dendrimers.145 Copyright permission from John Wiley and Sons.](/image/article/2013/CS/c3cs60101c/c3cs60101c-f6.gif) | ||
| Fig. 6 (a) Arylthiophene cored [G4] bis-MPA dendrimers for optical-power limiting (OPL) applications,106 (b) ordered honeycomb membranes from core crosslinked star AB block-copolymer hybrids consisting of PS and bis-MPA dendrons55 and (c) ultrathin films via LbL methodology from monodisperse bis-MPA dendrimers.145 Copyright permission from John Wiley and Sons. | ||
b. Biological
Today dendritic structures based on bis-MPA are intensively considered as promising macromolecular probes in biological research.34 This capitalizes on their aliphatic polyester structure, yielding a biocompatible and hydrolytically degradable architecture.110 Today, a large number of bioactive substituents have successfully been covalently attached to these scaffolds including carbohydrates,16,33,111 peptides,33,111 anticancer doxorubicin (DOX),31,112,113 and antibiotic penicillin derivative amoxicillin.28 To accomplish accurate postfunctionalizations, the peripheral hydroxyl groups are converted to reactive intermediates including alkenes,27,28,111 acetylenes24,32,33 and p-nitrophenyl carbonates.112,114 The latter resulted in facile introduction of both polyethylene glycol chains115 and DOX, with pH-sensitive hydrazone linkages,113 into a library of HFDs, Fig. 4d. In cell culture, a carefully selected HFD drug delivery vehicle with the nomenclature17 HFD(e)-G3-(PEO5k)8-b-G4-(DOX)16 was found to be more than 10 times less toxic than free DOX. Furthermore, the efficacy of the carrier, containing 20 mg kg−1 DOX, on albino house mouse (BALB/c)e bearing C-26 tumors via intravenous administration was found to exhibit a nine fold higher accumulation in tumor than free DOX after 48 h. Remarkably, a single injection of the DOX loaded HFD carrier, at 20 mg kg−1 DOX equivalents 8 days after tumor implantation, caused complete tumor regression and 100% survival of the mice.113 A heterofunctional dendrimer decorated with sixteen mannose units and two coumarin chromophores, HFD(e)-G4-(mannose)16-b-G1-(coumarin)2, was evaluated by Hawker et al. as a recognition/detection agent for the inhibition of hemagglutination. The multivalent representation of the carbohydrates was found to exhibit 240-fold greater potency than monomeric mannose i.e. a relative activity of 15 per sugar group when compared to mono-mannose.Since hydroxyl-functional hyperbranched polyesters have a significant resemblance to the corresponding dendrimers they have also been evaluated in biological applications. A blocking agent based on Boltorn H30, pseudo [G3], with mannose peripheral groups suggested inhibition at nanomolar concentrations between cell-specific intercellular adhesion molecular 3-grabbling nonintegrin (DC-SIGN) and Ebola virus (EBOV).116,117 Dual functional hyperbranched polyester based on Boltorn H2003 has also been accomplished to introduce combinations of fluorescein, folic acid and MTX for use as a drug delivery vehicle.118 One area where the interest for hyperbranched polymers has emerged significantly is encapsulation of dyes or drug moieties.119 The structural characteristics of hyperbranched polymers, their low viscosity and their relatively low price make them potential candidates for successful encapsulation applications. Karatsos et al. have modeled Boltorn H20's and H30's capability to host the pharmaceutical molecule ‘Shikonon’ using fully atomistic molecular dynamics simulation.120 It was found that a higher pseudo-generation gave rise to a more efficient drug loading and that conformational characteristics and the solution's concentration may affect the polymer–drug complexation. A higher loading capacity is interpreted as an effect of increased hydrogen bonding capacity.
In more recent work by Nyström et al., Boltorn H30 and H40 were modified with an average of 5 PEG chains (5 or 10 kDa) prior to the encapsulation of DOX. The resulting constructs were evaluated as passive drug delivery vehicles and found to exhibit enhanced drug efficacy and a higher degree of apoptosis compared to free drug.121 These Boltorn based drug delivery systems were taken up via clathrin- and macropinocytosis-mediated endocytosis.122 The same group successfully encapsulated DOX in PCL and PEG containing linear-dendritic hybrids.123 The resulting self-assembled micelles were approximately 100 nm in diameter and capable of achieving DOX loading efficiencies of up to 22 wt%. The loading amount of DOX was largely influenced by the size of the dendron. The encapsulation of DOX was also successful in polymer based theranostic systems, built from non-toxic star-like dendritic-linear polymer hybrids consisting of [G1] and [G2] bis-MPA dendrimers decorated with hydrophilic and fluorinated poly(PEGMA-co-TFMA) chains. With larger hydrophobic dendrimer cores, the release kinetics of DOX could be tailored revealing cellular uptake of the DOX loaded micelles into breast cancer cells.124 Gillies et al. have also elegantly reported on the introduction of dendritic blocks comprising rhodamine and Gd(III) chelates to PCL-PEG based micelles.125,126 The Gd(III) surface functionalized micelles were proposed as high-relaxivity MRI contrast agents. In an alternative approach, superparamagnetic iron oxide (SPIO) nanoparticles were decorated with [G3] dendrons comprising guanidine end groups.127 The dendrons displayed enhanced uptake of SPIO into GL261 mouse glioma cells.
c. Thin films and crosslinked networks
The branched architectures expressing a large number of functional groups and low viscosity in solution have enabled the utilization of dendritic polymers in coating applications. In thin film applications, the hyperbranched bis-MPA polymers are the most assessed and reviewed.128,129 The peripheral exposed functional groups of the dendritic scaffolds eliminate the formation of chain entanglements which is crucial for efficient film formation.Dendronized Au surfaces with monolayer of bis-MPA dendrons of different generation and PEG chain length were also evaluated by Adronov et al. with respect to protein resistance, cell adhesion and proliferation.96–98 The protein resistance increased with increasing generation of dendrons and increasing chain length of PEG to 2 kDa. The PEG-functionalized dendronized surfaces exhibited a significant reduction in cell growth, whereas the hydroxyl functional dendronized surfaces showed a considerably higher affinity for the cells. A potential biosensor with self-assembled dendritic monolayer (SADM) of bis-MPA dendrons that display mannose carbohydrates was also evaluated, by Malkoch and Berglin et al., for their multivalent interaction and scavenging ability towards Escherichia coli MS7fim+ bacteria.90 The carbohydrate decorated sensors based on [G3] dendrons revealed 2.5 times enhanced recognition of bacteria, when compared to hydroxyl functional surfaces. The same group also explored bifunctional dendronized cellulose surfaces as biosensors.92 A 10-fold stronger multivalency to lectin protein Concanavalin A was detected for the biosensors based on [G5] bis-MPA dendrons displaying multiple mannose substituents when compared to the monomeric counterpart. Self-assembled arrays of [G4] carboxylated bis-MPA dendrimers onto gold surfaces were accomplished with a defined separation of 17 or 28 nm.146 The surfaces were found to enhance the growth of endothelial cells and binding of proteins from the cell media when compared to commercially available poly(acrylic acid) of the same molecular weight.
4. Commercial availability
All the bis-MPA based dendritic architectures described here have academically been reported supported by detailed experimental protocols. As a result, an experienced dendrimer or polymer chemist can reproduce many of the elegant examples found in the literature. However, for the broad scientific community with limited knowledge in specific synthesis of bis-MPA based dendritic structures, the commercial route is an alternative to facilitate their evaluation in desired cutting-edge research. Today, the producers Perstorp AB and Polymer Factory Sweden AB along with the distributor Sigma Aldrich Corporation are three global providers of a selection of these scaffolds, Table 2.| Bis-MPA based dendritic scaffolds | Perstorp | Polymer Factory | Sigma-Aldrich |
|---|---|---|---|
| a Large scale availability for customers with final applications. | |||
| Monomers | × | × | |
| HBP | ×a | × | × |
| Dendrons | × | × | |
| Homofunctional dendrimers | × | ||
| Heterofunctional dendrimer | × | ||
| Dendritic-linear hybrids | × | × | |
| Tailor-made dendritic polymers | × | ||
5. Summary and outlook
Bis-MPA based dendritic materials belong to one of the most utilized dendritic families. This is ultimately related to their facile synthesis and hybridization using most known polymerization techniques. As a result, a remarkable number of sophisticated architectures have successfully been accomplished including monodisperse dendrons and dendrimers as well as polydisperse hyperbranched and dendritic-linear hybrids. The introduction of the click concept has further expanded the number of suggested bis-MPA dendritic architectures and active substituents by benign postfunctionalization to include optical and bioactive groups. With the commercial availability and excellent biocompatibility, researchers are presently exploring these frameworks for a wide variety of applications. However, the use of suggested new polymeric materials for in vivo medicinal applications is not straightforward since that has to pass the hurdle of various clinical phases. To the best of our knowledge, FDA or other entities have not yet approved any dendritic carrier based on bis-MPA for use as a drug delivery vehicle.Acknowledgements
The Swedish Research Council (VR) is gratefully acknowledged for financial support (M.M. grant 2010-453). Marie V. Walter, Vilhelm Olsson and Samuel Pendergraph are thanked for their aid with the review.References
- P. J. Flory, J. Am. Chem. Soc., 1952, 74, 2718–2723 CrossRef CAS
.
-
Dendrimers and Other Dendritic Polymers, ed. J. M. J. Fréchet and D. A. Tomalia, Wiley, Chichester, 2001 Search PubMed
.
-
G. R. Newkome, C. N. Moorefield and F. Vögtle, Dendrimers and Dendrons: Concepts, Syntheses, Applications, Wiley-VCH, Weinheim, 2001 Search PubMed
.
-
F. Vögtle, G. Richardt, N. Werner and A. J. Rackstraw, Dendrimer Chemistry: Concepts, Syntheses, Properties, Applications, Wiley-VCH, Weinheim, 2009 Search PubMed
.
- E. Buhleier, W. Wehner and F. Vögtle, Synthesis, 1978, 155–158 CrossRef CAS
.
- D. A. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117–132 CrossRef CAS
.
- G. R. Newkome, Z. Q. Yao, G. R. Baker and V. K. Gupta, J. Org. Chem., 1985, 50, 2003–2004 CrossRef CAS
.
- B. I. Voit and A. Lederer, Chem. Rev., 2009, 109, 5924–5973 CrossRef CAS
.
- B. Voit, J. Polym. Sci., Polym. Chem. Ed., 2005, 43, 2679–2699 CrossRef CAS
.
- C. Gao and D. Yan, Prog. Polym. Sci., 2004, 29, 183–275 CrossRef CAS
.
- M. Johansson, E. Malmstrom and A. Hult, J. Polym. Sci., Polym. Chem. Ed., 1993, 31, 619–624 CrossRef CAS
.
- E. Malmstrom, M. Johansson and A. Hult, Macromolecules, 1995, 28, 1698–1703 CrossRef
.
- E. Zagar and M. Zigon, Prog. Polym. Sci., 2011, 36, 53–88 CrossRef CAS
.
- H. Ihre, A. Hult and E. Soderlind, J. Am. Chem. Soc., 1996, 118, 6388–6395 CrossRef CAS
.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS
.
- P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. J. Fréchet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928–3932 CrossRef CAS
.
- M. V. Walter and M. Malkoch, Chem. Soc. Rev., 2012, 41, 4593–4609 RSC
.
- A. Carlmark, C. J. Hawker, A. Hult and M. Malkoch, Chem. Soc. Rev., 2009, 38, 352–362 RSC
.
- H. Ihre, A. Hult, J. M. J. Fréchet and I. Gitsov, Macromolecules, 1998, 31, 4061–4068 CrossRef CAS
.
- H. Ihre, O. L. P. De Jesus and J. M. J. Fréchet, J. Am. Chem. Soc., 2001, 123, 5908–5917 CrossRef CAS
.
- M. Malkoch, E. Malmstrom and A. Hult, Macromolecules, 2002, 35, 8307–8314 CrossRef CAS
.
- M. V. Walter, P. Lundberg, A. Hult and M. Malkoch, J. Polym. Sci., Polym. Chem. Ed., 2011, 49, 2990–2995 CrossRef CAS
.
- G. Franc and A. Kakkar, Chem. Commun., 2008, 5267–5276 RSC
.
- M. Malkoch, K. Schleicher, E. Drockenmuller, C. J. Hawker, T. P. Russell, P. Wu and V. V. Fokin, Macromolecules, 2005, 38, 3663–3678 CrossRef CAS
.
- P. Antoni, D. Nystrom, C. J. Hawker, A. Hult and M. Malkoch, Chem. Commun., 2007, 2249–2251 RSC
.
- K. L. Killops, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 5062–5064 CrossRef CAS
.
- M. I. Montanez, L. M. Campos, P. Antoni, Y. Hed, M. V. Walter, B. T. Krull, A. Khan, A. Hult, C. J. Hawker and M. Malkoch, Macromolecules, 2010, 43, 6004–6013 CrossRef CAS
.
- P. Antoni, M. J. Robb, L. Campos, M. Montanez, A. Hult, E. Malmstrom, M. Malkoch and C. J. Hawker, Macromolecules, 2010, 43, 6625–6631 CrossRef CAS
.
- A. Vieyres, T. Lam, R. Gillet, G. Franc, A. Castonguay and A. Kakkar, Chem. Commun., 2010, 46, 1875–1877 RSC
.
- X. P. Ma, Z. X. Zhou, E. L. Jin, Q. H. Sun, B. Zhang, J. B. Tang and Y. Q. Shen, Macromolecules, 2013, 46, 37–42 CrossRef CAS
.
- E. R. Gillies and J. M. J. Fréchet, J. Am. Chem. Soc., 2002, 124, 14137–14146 CrossRef CAS
.
- P. Wu, M. Malkoch, J. N. Hunt, R. Vestberg, E. Kaltgrad, M. G. Finn, V. V. Fokin, K. B. Sharpless and C. J. Hawker, Chem. Commun., 2005, 5775–5777 RSC
.
- P. A. Ledin, F. Friscourt, J. Guo and G. J. Boons, Chem.–Eur. J., 2011, 17, 839–846 CrossRef CAS
.
- C. C. Lee, J. A. MacKay, J. M. J. Fréchet and F. C. Szoka, Nat. Biotechnol., 2005, 23, 1517–1526 CrossRef CAS
.
- A. P. Goodwin, S. S. Lam and J. M. J. Fréchet, J. Am. Chem. Soc., 2007, 129, 6994–6995 CrossRef CAS
.
- I. Gitsov, K. L. Wooley and J. M. J. Fréchet, Angew. Chem., 1992, 104, 1282–1285 CrossRef CAS
(See also Angew. Chem., Int. Ed. Engl., 1992, 1231 (1289), 1200–1282).
- I. Gitsov, K. L. Wooley, C. J. Hawker and J. M. J. Fréchet, Polym. Prepr., 1991, 32, 631–632 CAS
.
- I. Gitsov, K. L. Wooley, C. J. Hawker, P. T. Ivanova and J. M. J. Fréchet, Macromolecules, 1993, 26, 5621–5627 CrossRef CAS
.
- I. Gitsov, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5295–5314 CrossRef CAS
.
- M. Trollsas, J. L. Hedrick, D. Mecerreyes, P. Dubois, R. Jerome, H. Ihre and A. Hult, Macromolecules, 1997, 30, 8508–8511 CrossRef CAS
.
- B. Atthoff, M. Trollsås, H. Claesson and J. L. Hedrick, Macromol. Chem. Phys., 1999, 200, 1333–1339 CrossRef CAS
.
- M. Trollsås, H. Claesson, B. Atthoff, J. L. Hedrick, J. A. Pople and A. P. Gast, Macromol. Symp., 2000, 153, 87–108 CrossRef
.
- H. Claesson, E. Malmström, M. Johansson and A. Hult, Polymer, 2002, 43, 3511–3518 CrossRef CAS
.
- A. Heise, J. L. Hedrick, M. Trollsås, R. D. Miller and C. W. Frank, Macromolecules, 1999, 32, 231–235 CrossRef CAS
.
- A. Heise, J. L. Hedrick, C. W. Frank and R. D. Miller, J. Am. Chem. Soc., 1999, 121, 8647–8648 CrossRef CAS
.
- A. Heise, C. Nguyen, R. Malek, J. L. Hedrick, C. W. Frank and R. D. Miller, Macromolecules, 2000, 33, 2346–2354 CrossRef CAS
.
- D. E. Poree, M. D. Giles, L. B. Lawson, J. He and S. M. Grayson, Biomacromolecules, 2011, 12, 898–906 CrossRef CAS
.
- X. Hao, C. Nilsson, M. Jesberger, M. H. Stenzel, E. Malmström, T. P. Davis, E. Östmark and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5877–5890 CrossRef CAS
.
- M. Jesberger, L. Barner, M. H. Stenzel, E. Malmström, T. P. Davis and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3847–3861 CrossRef CAS
.
- X. Zeng, Y. Zhang, Z. Wu, P. Lundberg, M. Malkoch and A. M. Nyström, J. Polym. Sci., Part A: Polym. Chem., 2011, 50, 280–288 CrossRef
.
- Y. J. Li, B. Y. Zhang, J. N. Hoskins and S. M. Grayson, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1086–1101 CrossRef CAS
.
- J. del Barrio, L. Oriol, R. Alcala and C. Sanchez, Macromolecules, 2009, 42, 5752–5760 CrossRef CAS
.
- H. Altin, I. Kosif and R. Sanyal, Macromolecules, 2010, 43, 3801–3808 CrossRef CAS
.
- S.-H. Yim, J. Huh, C.-H. Ahn and T. G. Park, Macromolecules, 2007, 40, 205–210 CrossRef CAS
.
- L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Adv. Funct. Mater., 2008, 18, 3706–3714 CrossRef CAS
.
- R. Vestberg, A. M. Piekarski, E. D. Pressly, K. Y. Van Berkel, M. Malkoch, J. Gerbac, N. Ueno and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1237–1258 CrossRef CAS
.
- A. Wuersch, M. Moeller, T. Glauser, L. S. Lim, S. B. Voytek, J. L. Hedrick, C. W. Frank and J. G. Hilborn, Macromolecules, 2001, 34, 6601–6615 CrossRef CAS
.
- T. Glauser, C. M. Stancik, M. Moeller, S. Voytek, A. P. Gast and J. L. Hedrick, Macromolecules, 2002, 35, 5774–5781 CrossRef CAS
.
- L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Macromolecules, 2007, 40, 7855–7863 CrossRef CAS
.
- P. Lundberg, M. V. Walter, M. I. Montanez, D. Hult, A. Hult, A. Nystroem and M. Malkoch, Polym. Chem., 2011, 2, 394–402 RSC
.
- E. R. Gillies and J. M. J. Fréchet, J. Org. Chem., 2004, 69, 46–53 CrossRef CAS
.
- H. Frauenrath, Prog. Polym. Sci., 2005, 30, 325–384 CrossRef CAS
.
- A. Zhang, L. Shu, Z. Bo and A. D. Schlüter, Macromol. Chem. Phys., 2003, 204, 328–339 CrossRef CAS
.
- A. D. Schlüter and J. P. Rabe, Angew. Chem., Int. Ed., 2000, 39, 864–883 CrossRef
.
- A. D. Schlüter, Top. Curr. Chem., 2005, 245, 151–191 Search PubMed
.
- R. Freudenberger, W. Claussen, A. D. Schluter and H. Wallmeier, Polymer, 1994, 35, 4496–4501 CrossRef CAS
.
- V. Percec, J. Heck, D. Tomazos, F. Falkenberg, H. Blackwell and G. Ungar, J. Chem. Soc., Perkin Trans. 1, 1993, 2799–2811 RSC
.
- J. I. Paez, M. Martinelli, V. Brunetti and M. C. Strumia, Polymers, 2012, 4, 355–395 CrossRef
.
- L. Shu, A. Schaefer and A. D. Schlueter, Macromolecules, 2000, 33, 4321–4328 CrossRef CAS
.
- A. D. Schluter, C. R. Chim., 2003, 6, 843–851 CrossRef CAS
.
- S. M. Grayson and J. M. J. Fréchet, Macromolecules, 2001, 34, 6542–6544 CrossRef CAS
.
- S. R. Benhabbour and A. Adronov, ACS Symp. Ser., 2008, 977, 238–249 CrossRef CAS
.
- M. Malkoch, A. Carlmark, A. Woldegiorgis, A. Hult and E. E. Malmström, Macromolecules, 2004, 37, 322–329 CrossRef CAS
.
- E. Östmark, J. Lindqvist, D. Nyström and E. Malmström, Biomacromolecules, 2007, 8, 3815–3822 CrossRef
.
- J. L. Mynar, T. L. Choi, M. Yoshida, V. Kim, C. J. Hawker and J. M. J. Fréchet, Chem. Commun., 2005, 5169–5171 RSC
.
- M. D. Giles, S. M. Liu, R. L. Emanuel, B. C. Gibb and S. M. Grayson, J. Am. Chem. Soc., 2008, 130, 14430–14431 CrossRef CAS
.
- Y. J. Li, M. D. Giles, S. M. Liu, B. A. Laurent, J. N. Hoskins, M. A. Cortez, S. G. Sreerama, B. C. Gibb and S. M. Grayson, Chem. Commun., 2011, 47, 9036–9038 RSC
.
- B. A. Laurent and S. M. Grayson, J. Am. Chem. Soc., 2011, 133, 13421–13429 CrossRef CAS
.
- A. Carlmark and E. E. Malmström, Macromolecules, 2004, 37, 7491–7496 CrossRef CAS
.
- S. Hietala, A. Nystrom, H. Tenhu and A. Hult, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3674–3683 CrossRef CAS
.
- A. Nyström and A. Hult, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 3852–3867 CrossRef
.
- A. M. Nyström, I. Furo, E. Malmström and A. Hult, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4496–4504 CrossRef
.
- A. Nyström, M. Malkoch, I. Furo, D. Nyström, K. Unal, P. Antoni, G. Vamvounis, C. Hawker, K. Wooley, E. Malmström and A. Hult, Macromolecules, 2006, 39, 7241–7249 CrossRef
.
- K. O. Kim and T.-L. Choi, ACS Macro Lett., 2012, 1, 445–448 CrossRef CAS
.
- A.-M. Zorn, M. Malkoch, A. Carlmark and C. Barner-Kowollik, Polym. Chem., 2011, 2, 1163–1173 RSC
.
- S. Peleshanko and V. V. Tsukruk, Prog. Polym. Sci., 2008, 33, 523–580 CrossRef CAS
.
- A. Sidorenko, X. W. Zhai, S. Peleshanko, A. Greco, V. V. Shevchenko and V. V. Tsukruk, Langmuir, 2001, 17, 5924–5931 CrossRef CAS
.
- H. Shulha, X. W. Zhai and V. V. Tsukruk, Macromolecules, 2003, 36, 2825–2831 CrossRef CAS
.
- E. Ostmark, L. Macakova, T. Auletta, M. Malkoch, E. Malmström and E. Blomberg, Langmuir, 2005, 21, 4512–4519 CrossRef
.
- K. Oberg, J. Ropponen, J. Kelly, P. Lowenhielm, M. Berglin and M. Malkoch, Langmuir, 2013, 29, 456–465 CrossRef
.
- D. A. Long, K. Unal, R. C. Pratt, M. Malkoch and J. Frommer, Adv. Mater., 2007, 19, 4471–4473 CrossRef CAS
.
- M. I. Montanez, Y. Hed, S. Utsel, J. Ropponen, E. Malmstroem, L. Wagberg, A. Hult and M. Malkoch, Biomacromolecules, 2011, 12, 2114–2125 CrossRef CAS
.
- P. Liu, Surf. Rev. Lett., 2005, 12, 619–622 CrossRef CAS
.
- P. Liu, Appl. Clay Sci., 2007, 35, 11–16 CrossRef CAS
.
- P. Liu and L. Zhang, Surf. Rev. Lett., 2007, 14, 1025–1032 CrossRef CAS
.
- R. Benhabbour Soumya, H. Sheardown and A. Adronov, Biomaterials, 2008, 29, 4177–4186 CrossRef CAS
.
- S. R. Benhabbour, L. Liu, H. Sheardown and A. Adronov, Macromolecules, 2008, 41, 2567–2576 CrossRef CAS
.
- S. R. Benhabbour, H. Sheardown and A. Adronov, Macromolecules, 2008, 41, 4817–4823 CrossRef CAS
.
- P. Busson, J. Ortegren, H. Ihre, U. W. Gedde, A. Hult and G. Andersson, Macromolecules, 2001, 34, 1221–1229 CrossRef CAS
.
- P. Busson, H. Ihre and A. Hult, J. Am. Chem. Soc., 1998, 120, 9070–9071 CrossRef CAS
.
- M. Rodlert, R. Vestberg, E. Malmstrom, M. Persson and M. Lindgren, Synth. Met., 2002, 127, 37–43 CrossRef CAS
.
- C. Pitois, A. Hult and M. Lindgren, J. Lumin., 2005, 111, 265–283 CrossRef CAS
.
- R. Vestberg, A. Nystrom, M. Lindgren, E. Malmstrom and A. Hult, Chem. Mater., 2004, 16, 2794–2804 CrossRef CAS
.
- R. Vestberg, R. Westlund, A. Eriksson, C. Lopes, M. Carlsson, B. Eliasson, E. Glimsdal, M. Lindgren and E. Malmstrom, Macromolecules, 2006, 39, 2238–2246 CrossRef CAS
.
- R. Westlund, E. Malmstrom, M. Hoffmann, R. Vestberg, C. Hawker, E. Glimsdal, M. Lindgren, P. Norman, A. Eriksson and C. Lopes, Optical Materials in Defence Systems Technology III, 2006, 6401, U87–U94.
- R. Vestberg, C. Nilsson, C. Lopes, P. Lind, B. Eliasson and E. Malmstrom, J. Polym. Sci., Polym. Chem. Ed., 2005, 43, 1177–1187 CrossRef CAS
.
- P. Antoni, M. Malkoch, G. Vamvounis, D. Nystrom, A. Nystrom, M. Lindgren and A. Hult, J. Mater. Chem., 2008, 18, 2545–2554 RSC
.
- P. A. Bouit, R. Westlund, P. Feneyrou, O. Maury, M. Malkoch, E. Malmstrom and C. Andraud, New J. Chem., 2009, 33, 964–968 RSC
.
- J. del Barrio, L. Oriol, R. Alcala and C. Sanchez, Macromolecules, 2009, 42, 5752–5760 CrossRef CAS
.
- N. Feliu, M. V. Walter, M. I. Montanez, A. Kunzmann, A. Hult, A. Nystrom, M. Malkoch and B. Fadeel, Biomaterials, 2012, 33, 1970–1981 CrossRef CAS
.
- M. Lo Conte, M. J. Robb, Y. Hed, A. Marra, M. Malkoch, C. J. Hawker and A. Dondoni, J. Polym. Sci., Polym. Chem. Ed., 2011, 49, 4468–4475 CrossRef
.
- O. L. P. De Jesus, H. R. Ihre, L. Gagne, J. M. J. Fréchet and F. C. Szoka, Bioconjugate Chem., 2002, 13, 453–461 CrossRef
.
- C. C. Lee, E. R. Gillies, M. E. Fox, S. J. Guillaudeu, J. M. J. Fréchet, E. E. Dy and F. C. Szoka, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 16649–16654 CrossRef CAS
.
- E. R. Gillies and J. M. J. Fréchet, J. Am. Chem. Soc., 2002, 124, 14137–14146 CrossRef CAS
.
- E. R. Gillies, E. Dy, J. M. J. Fréchet and F. C. Szoka, Mol. Pharmaceutics, 2005, 2, 129–138 CrossRef CAS
.
- F. Lasala, E. Arce, J. R. Otero, J. Rojo and R. Delgado, Antimicrob. Agents Chemother., 2003, 47, 3970–3972 CrossRef CAS
.
- E. Arce, P. M. Nieto, V. Diaz, R. G. Castro, A. Bernad and J. Rojo, Bioconjugate Chem., 2003, 14, 817–823 CrossRef CAS
.
- J. R. Wang and T. W. Xu, Polym. Adv. Technol., 2011, 22, 763–767 CrossRef CAS
.
- M. Irfan and M. Seiler, Ind. Eng. Chem. Res., 2010, 49, 1169–1196 CrossRef CAS
.
- I. Tanis, K. Karatasos, A. N. Assimopoulou and V. P. Papageorgiou, Phys. Chem. Chem. Phys., 2011, 13, 10808–10817 RSC
.
- X. H. Zeng, Y. N. Zhang, Z. H. Wu, P. Lundberg, M. Malkoch and A. M. Nystrom, J. Polym. Sci., Polym. Chem. Ed., 2012, 50, 280–288 CrossRef CAS
.
- X. H. Zeng, Y. N. Zhang and A. M. Nystrom, Biomacromolecules, 2012, 13, 3814–3822 CrossRef CAS
.
- Z. H. Wu, X. H. Zeng, Y. N. Zhang, N. Feliu, P. Lundberg, B. Fadeel, M. Malkoch and A. M. Nystrom, J. Polym. Sci., Polym. Chem. Ed., 2012, 50, 217–226 CrossRef CAS
.
- C. Porsch, Y. Zhang, Å. Östlund, P. Damberg, C. Ducani, E. Malmström and A. M. Nyström, Part. Part. Syst. Charact., 2013, 30, 381–390 CrossRef CAS
.
- A. Nazemi, R. C. Amos, C. V. Bonduelle and E. R. Gillies, J. Polym. Sci., Polym. Chem. Ed., 2011, 49, 2546–2559 CrossRef CAS
.
- A. Nazemi, F. Martinez, T. J. Scholl and E. R. Gillies, RSC Adv., 2012, 2, 7971–7973 RSC
.
- A. L. Martin, L. M. Bernas, B. K. Rutt, P. J. Foster and E. R. Gillies, Bioconjugate Chem., 2008, 19, 2375–2384 CrossRef CAS
.
-
B. Bruchmann and B. Voit, Applications of Hyperbranched Polymers in Coatings, as Additives, and in Nanotechnology, John Wiley & Sons Inc., Hoboken, 2011 Search PubMed
.
- X. L. Zhang, Polym. Int., 2011, 60, 153–166 CrossRef CAS
.
- L. H. Hu, A. Asif, J. D. Xie and W. F. Shi, Polym. Adv. Technol., 2011, 22, 1673–1680 CrossRef CAS
.
- J. J. Decker, S. N. Chvalun and S. Nazarenko, Polymer, 2011, 52, 3943–3955 CrossRef CAS
.
- L. Fogelstrom, E. Malmstrom, M. Johansson and A. Hult, ACS Appl. Mater. Interfaces, 2010, 2, 1679–1684 Search PubMed
.
- M. Rodlert, C. J. G. Plummer, L. Garamszegi, Y. Leterrier, H. J. M. Grunbauer and J. A. E. Manson, Polymer, 2004, 45, 949–960 CrossRef CAS
.
- M. Rodlert, C. J. G. Plummer, H. J. M. Grunbauer and J. E. Manson, Adv. Eng. Mater., 2004, 6, 715–719 CrossRef CAS
.
- M. Rodlert, C. J. G. Plummer, Y. Leterrier, J. A. E. Manson and H. J. M. Grunbauer, J. Rheol., 2004, 48, 1049–1065 CrossRef CAS
.
- J. W. Bartels, P. M. Imbesi, J. A. Finlay, C. Fidge, J. Ma, J. E. Seppala, A. M. Nystrom, M. E. Mackay, J. A. Callow, M. E. Callow and K. L. Wooley, ACS Appl. Mater. Interfaces, 2011, 3, 2118–2129 CAS
.
- M. Lomba, L. Oriol, R. Alcala, C. Sanchez, M. Moros, V. Grazu, J. L. Serrano and J. M. De la Fuente, Macromol. Biosci., 2011, 11, 1505–1514 CrossRef CAS
.
- X. Z. Wei, J. Yang, Y. Y. Xu, B. K. Zhu and G. L. Zhang, J. Porous Mater., 2012, 19, 721–731 CrossRef CAS
.
- X. Z. Wei, L. P. Zhu, H. Y. Deng, Y. Y. Xu, B. K. Zhu and Z. M. Huang, J. Membr. Sci., 2008, 323, 278–287 CrossRef CAS
.
- X. Z. Wei, J. Yang and G. L. Zhang, Polym. Polym. Compos., 2012, 20, 261–270 CAS
.
- J. Zou, Y. Zhao, W. Shi, X. Shen and K. Nie, Polym. Adv. Technol., 2005, 16, 55–60 CrossRef CAS
.
- M. V. Walter, P. Lundberg, D. Hult, A. Hult and M. Malkoch, Polym. Chem., 2013, 4, 2680–2690 RSC
.
- L. L. Wang, Z. L. Meng, Y. L. Yu, Q. W. Meng and D. Z. Chen, Polymer, 2008, 49, 1199–1210 CrossRef CAS
.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339–343 CrossRef CAS
.
- R. Vestberg, M. Malkoch, M. Kade, P. Wu, V. V. Fokin, K. B. Sharpless, E. Drockenmuller and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2835–2846 CrossRef CAS
.
- A. Lundgren, Y. Hed, K. Oeberg, A. Sellborn, H. Fink, P. Lowenhielm, J. Kelly, M. Malkoch and M. Berglin, Angew. Chem., Int. Ed., 2011, 50, 3450–3453 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |