Effect of tight flavin mononucleotide wrapping and its binding affinity on carbon nanotube covalent reactivities†
Abstract
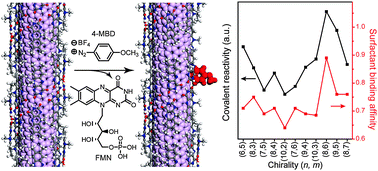
The controlled functionalization of single-walled carbon nanotubes (SWNTs) is a key to using them in high-end applications. We show that nanotube reactivity after covalent diazonium modification is governed by a chirality-specific surfactant binding affinity to SWNTs. Both metallic and semiconducting SWNTs tightly organized by a helical flavin mononucleotide (FMN) assembly exhibit two hundred times slower reactivity toward 4-methoxy benzenediazonium (4-MBD) than those wrapped by sodium dodecyl sulfate and this reactivity enables chirality- and metallicity-specific behaviours to be probed, as confirmed by absorption, Raman, and photoluminescence (PL) spectroscopy. Each reaction kinetic of the two-step SWNT PL decays originating from electron transfer and the covalent reaction of 4-MBD, respectively, is inversely proportional to the binding affinity (Ka) between FMN and the SWNTs. The observed marginally higher reaction rate of the metallic nanotube compared to the semiconducting one results from the weaker Ka value of the metallic nanotubes with FMN. An enrichment demonstration of a few nanotube chiralities using selective and slow covalent diazonium chemistry demonstrates the importance of the binding affinity between the surfactant and the SWNTs. The study provides a handle on chirality-specific covalent chemistry via surfactant–SWNT binding affinity and impacts on future-sensing schemes.

 Please wait while we load your content...
Please wait while we load your content...