Crystallisation of solvothermally synthesised ZIF-8 investigated at the bulk, single crystal and surface level†
Abstract
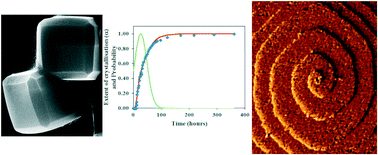
A gravimetric study of the solvothermal dimethylformamide-mediated crystallisation of the metal–organic framework ZIF-8 has enabled the rate constants and activation energies of the nucleation and crystal growth processes to be determined. The kinetics analyses reveal the crystallisation is nucleation controlled with activation energies for nucleation and crystal growth of 115.1 and 87.7 kJ mol−1, respectively. The results are compared to the crystallisation kinetics for other syntheses of ZIF-8 and other MOFs to reveal the importance that different solvents, different metal ion/organic linker ratios and the presence of deprotonating modulators have on these processes. Temporal ex situ monitoring of the crystal morphology and surface topography during crystallisation reveals the crystals evolve to the equilibrium rhombic dodecahedral morphology via edge-truncated cubic and corner-truncated rhombic dodecahedral habits while relatively smooth surfaces containing large truncated rhombic surface terraces and growth spirals develop through rough surfaces consisting of numerous ill-defined small growth islands and steps. The combination of techniques applied over different size scales provides important understanding for potential control over the crystal properties of MOFs for new application or enhanced performance in current applications.
- This article is part of the themed collection: Structural Design of Coordination Polymers

 Please wait while we load your content...
Please wait while we load your content...