Two-step crystal nucleation via capillary condensation
Abstract
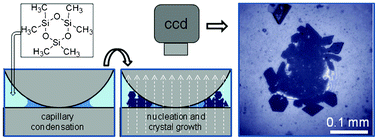
There are many examples where classical nucleation theory does not provide a correct description of the formation of a crystal from a solution. Many of these examples have been grouped together under the term “two-step mechanisms of crystal nucleation”, because the common denominator is the initial formation of a denser, often metastable fluid phase, followed by nucleation of the crystal from this precursor. A number of well established routes to crystallisation, such as those involving amorphous precursor phases to biominerals like calcite and hydroxyapatite would fit into this category, as would the many recently discovered cases of protein nucleation from dense fluid clusters of molecules. We here discuss a two-step mechanism that has been experimentally identified in heterogeneous crystal nucleation from vapour. In this process, supercooled liquid condenses from vapour in surface cavities and then freezes, most likely via homogeneous nucleation. As a result, crystal deposition may under favourable conditions occur from saturated vapour with no need whatsoever for supersaturation. These results are of great potential significance for atmospheric ice nucleation, and similar ideas were advanced almost 50 years ago, but were never experimentally tested. Thermodynamic analogies with solution crystallisation lead us to suggest that heterogeneous nucleation in solution might in many cases proceed by a similar route, as has been found in computer simulation studies of protein nucleation in surface pits.

 Please wait while we load your content...
Please wait while we load your content...