 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evidence that the catenane form of CS2 hydrolase is not an artefact†
Mark B. van Eldijka, Iris van Leeuwenab, Victor A. Mikhailovc, Lotte Neijenhuisab, Harry R. Harhangib, Jan C. M. van Hesta, Mike S. M. Jettenb, Huub J. M. Op den Campb, Carol V. Robinsonc and Jasmin Mecinović*a
aInstitute for Molecules and Materials, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands. E-mail: j.mecinovic@science.ru.nl; Fax: +31 24 3653393; Tel: +31 24 3652381
bDepartment of Microbiology, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands
cChemistry Research Laboratory, University of Oxford, Mansfield Road, OX1 3TA Oxford, UK
First published on 3rd June 2013
Abstract
CS2 hydrolase, a zinc-dependent enzyme that converts carbon disulfide to carbon dioxide and hydrogen sulfide, exists as a mixture of octameric ring and hexadecameric catenane forms in solution. A combination of size exclusion chromatography, multi-angle laser light scattering, and mass spectrometric analyses revealed that the unusual catenane structure is not an artefact, but a naturally occurring structure.
In the last couple of decades, chemistry has witnessed a flourishing field of mechanically interlocked structures, including catenanes. Well established synthetic pathways have facilitated the preparation of diverse sets of catenane architectures which have found an important place in material sciences as molecular switches, electronic devices and sensors.1,2
Catenane structures in more complex biological systems, however, have been found to be rare.3 For instance, catenane DNA structures have been detected inside living cells.4,5 To date, only a few proteins have been observed to exist in catenane forms: (i) a bacteriophage HK97 capsid in which the catenane structure is maintained via a covalent isopeptide bond between Lys169 and Asn356,6,7 (ii) a thermophile Pyrobaculum aerophilum citrate synthase (PaCS) in which the disulfide formation leads to catenation,8 (iii) a Cys168Ser variant of mitochondrial peroxiredoxin III,9 (iv) RecR, an enzyme involved in the DNA repair,10 and (v) E. coli Ia class of ribonucleotide reductase (RNR).11
CS2 hydrolase is a zinc-dependent enzyme from the thermophilic archaeon Acidianus A1-3 that converts carbon disulfide to carbon dioxide and hydrogen sulfide.12 It exhibits a high degree of homology to β-carbonic anhydrase, and contains a 2Cys-His catalytic triad for the chelation of zinc ions. Recent X-ray crystallographic, small angle X-ray scattering, analytical ultracentrifugation, and native gel data revealed that CS2 hydrolase exists as a mixture of the octameric ring and the hexadecameric catenane structures, both in the crystal and in solution (Fig. 1).12 In the compact catenane form, two octameric rings are positioned perpendicularly. The octameric ring and the hexadecameric catenane structures are both maintained via non-covalent interactions.
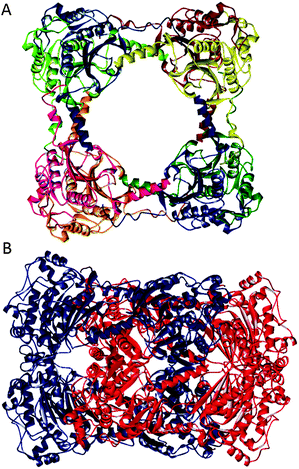 | ||
| Fig. 1 Octameric ring (A) and hexadecameric catenane (B) forms of CS2 hydrolase (PDB ID: 3TEN, 3TEO). | ||
Although catenane architectures were observed for RecR10 and Ia class of RNR11 by X-ray crystallography, solution data indicated that the catenane forms existed only under very specific experimental conditions (i.e. at higher protein concentration for RecR, and at higher concentration of the precipitant that contains polyethylene glycol for RNR). In this regard, the catenane forms of these two proteins were the result of high concentration of the protein or the crystallisation precipitant, and thus considered as an artefact. Herein, we describe that CS2 hydrolase truely exists in the form of the catenane, and that the interlocked structure is not an artefact.
In order to verify that CS2 hydrolase forms the catenane topology under diverse sets of experimental conditions, we focused especially on detecting it in the range of concentrations of the protein in the absence of precipitating agents. Despite a significant difference of molecular weights of the ring (189.1 kDa) and the catenane (378.3 kDa) forms of CS2 hydrolase, a good separation of these forms of CS2 hydrolase has been found to be difficult using size exclusion chromatography (SEC). The difficulty with purification by SEC is presumably a result of a smaller difference (i.e. less than two fold) in the volume of both species, as illustrated by their crystal structures (Fig. 1). Therefore, purified mixtures of the ring and the catenane were further evaluated at different concentrations in HEPES buffer (20 mM, pH 7.5). Notably, a ratio of approximately 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between the octameric ring and the hexadecameric catenane forms remained the same in the series of dilutions (concentrations of the loaded protein samples were between 8.0 mg mL−1 and 0.08 mg mL−1; Fig. 2A and B, see also Fig. S1 and S2 in ESI†). Due to the dilution of the sample during SEC, we note that the actual concentration of the protein on the column is on average approximately 20-fold lower than that of the loaded one. These data clearly illustrate that the catenane form of CS2 hydrolase is not a consequence of the highly concentrated protein, but ultimately a form that is stable in very dilute solutions. Moreover, the ratios between both oligomeric assemblies remained indistinguishable in Tris buffer (at 20 mM, pH 7.5, Fig. 2C).
1 between the octameric ring and the hexadecameric catenane forms remained the same in the series of dilutions (concentrations of the loaded protein samples were between 8.0 mg mL−1 and 0.08 mg mL−1; Fig. 2A and B, see also Fig. S1 and S2 in ESI†). Due to the dilution of the sample during SEC, we note that the actual concentration of the protein on the column is on average approximately 20-fold lower than that of the loaded one. These data clearly illustrate that the catenane form of CS2 hydrolase is not a consequence of the highly concentrated protein, but ultimately a form that is stable in very dilute solutions. Moreover, the ratios between both oligomeric assemblies remained indistinguishable in Tris buffer (at 20 mM, pH 7.5, Fig. 2C).
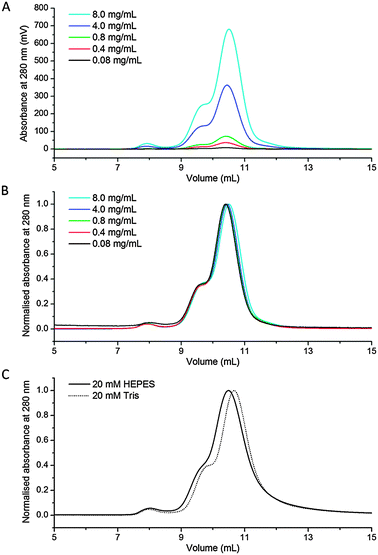 | ||
| Fig. 2 (A) SEC of CS2 hydrolase at 8.0 mg mL−1, 4.0 mg mL−1, 0.8 mg mL−1, 0.4 mg mL−1 and 0.08 mg mL−1; (B) normalised chromatograms; (C) normalised chromatograms in HEPES and Tris buffer. | ||
Multi-angle laser light scattering (MALLS) analysis of the purified mixture of the ring and the catenane further proved that the observed molecular weights of both species are in good agreement with calculated ones (180 kDa for the ring, 340 kDa for the catenane, Fig. 3A and Fig. S3, ESI†). Furthermore, native PAGE gels also provided evidence that both peaks observed in the chromatogram correspond to the octameric ring and the hexadecameric catenane (Fig. 3B).
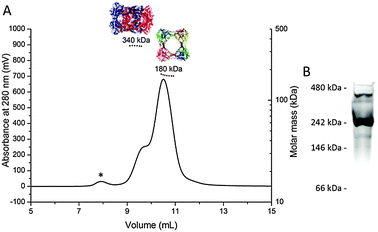 | ||
| Fig. 3 (A) SEC-MALLS chromatogram of CS2 hydrolase at 8.0 mg mL−1; (B) Coomassie-stained native PAGE analysis of CS2 hydrolase. The asterisk indicates a minor fraction of large aggregates. | ||
Having shown that the ring and the catenane forms are present in solution, we then investigated whether both structures can also be observed in the gas phase. Non-denaturing electrospray ionisation mass spectrometric (ESI-MS) analysis of the mixture of both species in ammonium acetate solution (200 mM, pH 7.5) showed that CS2 hydrolase is amenable for studies in the gas phase. Molecular weights of 188.9 kDa and 378.5 kDa were observed for the octameric ring and the hexadecameric catenane, respectively (Fig. S4, ESI†). Native ESI-MS demonstrated that the charge states of the octameric ring range from 28+ to 32+, and those of the hexadecameric catenane range from 39+ to 44+. These ranges remained the same upon dilution of the sample in ammonium acetate solution, i.e. upon reduction of the protein concentration from 2.0 mg mL−1 to 0.125 mg mL−1 (Fig. 4). Although the relative abundance of the charge states within these distributions slightly changed, and the peaks became narrower as the remaining molecules of the original HEPES buffer were ‘washed’ off from the complexes, the ratio between the octameric ring and hexadecameric catenane basically remained the same. The distribution of charge states for the ring form (30+ the most abundant) and the catenane form (40+ the most abundant) implies that about one third of the rings' surface in the catenane form is not accessible for ionisation, an observation that is compatible with crystallographic analysis of both forms.
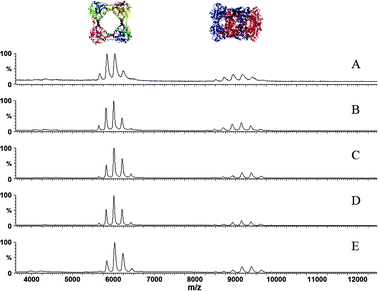 | ||
| Fig. 4 Non-denaturing ESI-MS studies of CS2 hydrolase showing that the ring form (m/z ∼ 6000) and the catenane form (m/z ∼ 9000) are present at different concentrations: (A) 2.0 mg mL−1; (B) 1.0 mg mL−1; (C) 0.5 mg mL−1; (D) 0.25 mg mL−1; (E) 0.125 mg mL−1. | ||
Overall, the results from mass spectrometric studies are in agreement with those from solution studies employing size exclusion chromatography; in both cases the ratio between the ring and the catenane is not perturbed by the overall concentration of the protein. Moreover, the results obtained using our three analytical techniques provide complementary information to X-ray crystallography about the existence of the ring and the catenane forms of CS2 hydrolase.
We have demonstrated that CS2 hydrolase is a special protein assembly in which the unusual catenane topology is maintained in both diluted and concentrated protein samples under various conditions. In addition to recent crystallographic and limited solution data,12 the advanced solution and gas phase experiments presented here provide clear evidence for the presence of the stable catenane form. In this regard, CS2 hydrolase seems to be an excellent, perhaps unique, protein model system for studies of biological catenanes.
We thank Radboud University Nijmegen, NWO, and STW for financial support.
Notes and references
- D. B. Amabilino and J. F. Stoddart, Chem. Rev., 1995, 95, 2725–2828 CrossRef CAS.
- C. Dietrich-Buchecker, M. C. Jimenez-Molero, V. Sartor and J. P. Sauvage, Pure Appl. Chem., 2003, 75, 1383–1393 CrossRef CAS.
- T. Yeates, T. S. Norcross and N. P. King, Curr. Opin. Chem. Biol., 2007, 11, 595–603 CrossRef CAS.
- D. A. Clayton and J. Vinograd, Nature, 1967, 216, 652–657 CrossRef CAS.
- B. Hudson and J. Vinograd, Nature, 1967, 216, 647–652 CrossRef CAS.
- C. Helgstrand, W. R. Wikoff, R. L. Duda, R. W. Hendrix, J. E. Johnson and L. Liljas, J. Mol. Biol., 2003, 334, 885–899 CrossRef CAS.
- W. R. Wikoff, L. Liljas, R. L. Duda, H. Tsuruta, R. W. Hendrix and J. E. Johnson, Science, 2000, 289, 2129–2133 CrossRef CAS.
- D. R. Boutz, D. Cascio, J. Whitelegge, L. J. Perry and T. O. Yeates, J. Mol. Biol., 2007, 368, 1332–1344 CrossRef CAS.
- Z. B. Cao, A. W. Roszak, L. J. Gourlay, J. G. Lindsay and N. W. Isaacs, Structure, 2005, 13, 1661–1664 CrossRef CAS.
- B. I. Lee, K. H. Kim, S. J. Park, S. H. Eom, H. K. Song and S. W. Suh, EMBO J., 2004, 23, 2029–2038 CrossRef CAS.
- C. M. Zimanyi, N. Ando, E. J. Brignole, F. J. Asturias, J. Stubbe and C. L. Drennan, Structure, 2012, 20, 1374–1383 CrossRef CAS.
- M. J. Smeulders, T. R. M. Barends, A. Pol, A. Scherer, M. H. Zandvoort, A. Udvarhelyi, A. F. Khadem, A. Menzel, J. Hermans, R. L. Shoeman, H. J. C. T. Wessels, L. P. van den Heuvel, L. Russ, I. Schlichting, M. S. M. Jetten and H. J. M. Op den Camp, Nature, 2011, 478, 412–416 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for expression and purification of CS2 hydrolase, native PAGE, size exclusion chromatography, multi-angle laser light scattering, mass spectrometric analysis and supplementary figures. See DOI: 10.1039/c3cc43219j |
| This journal is © The Royal Society of Chemistry 2013 |
