Gold nanoparticle research before and after the Brust–Schiffrin method
Luis M.
Liz-Marzán
abc
aDepartamento de Química Física, Universidade de Vigo, 36310 Vigo, Spain
bCentre for Cooperative Research in Biomaterials (CiC biomaGUNE), Paseo de Miramón 182, 20009 San Sebastián, Spain
cIkerbasque, Basque Foundation for Science, 48011 Bilbao, Spain. E-mail: llizmarzan@cicbiomagune.es; Fax: +34 943005301
First published on 3rd October 2012
Abstract
In this viewpoint we discuss the early contribution from Brust et al., which is not only the most cited paper ever published in Chem. Commun., but also a landmark in gold nanoparticle research and in nanotechnology. We provide here an overview of its major contributions and how the field has evolved since its publication in 1994.
Gold has traditionally been a material of choice for scientific research, as well as for a large number of technological applications, the main reason being its remarkable chemical stability and processability. Additionally, its metallic nature provides exceptionally attractive electronic and optical properties that have always called for attention. In the context of nanoscience, gold nanoparticles have also been long studied and used. According to the scientists at the British Museum, Romans fabricated glasses which were coloured by incorporation of metallic nanoparticles, as demonstrated in the ubiquitous example of the Lycurgus cup. Stained glass technology was further developed in the middle ages and was described by Johann Knuckel and others in the XVIIth century. Fabrication of the purple of Cassius and ruby red is usually cited as a milestone in this technology.1 However, the first scientific description of the preparation of gold colloids and discussion of their “action on light” are due to Michael Faraday, who presented his discoveries in a lecture at the Royal Institution in 1852.2 Faraday's work has been essential for the development of colloid science, which did not even have a name at that time. Names such as Zsigmondy or Perrin (Nobel laureates) have been long associated with colloidal gold for their discoveries of the properties of colloids.
The use of gold as a model system for the study of the properties of colloids, as well as early applications, for example in the field of electron microscopy for contrast enhancement, made it necessary to develop synthetic protocols that yielded gold nanocrystals with narrow size distributions. By far, the citrate reduction method reported by Turkevich and co-workers in the mid XXth century became the most popular and standard protocol for the synthesis of monodisperse aqueous gold colloids.3 However, although this method can be tuned to obtain quasi-spherical nanoparticles with average sizes ranging between 10 and 50 nm, smaller particles are very hard to obtain. Additionally, the surface chemistry of the obtained particles is poorly defined since citrate gets degraded during synthesis and the binding of citrate and its derivatives to the gold surface is of purely physical nature. When nanotechnology became a (more than) popular field (mainly after Feynman's lecture in 1959) and understanding size effects was an essential milestone, it was obvious that new chemical methods were needed that allowed the fabrication of smaller particles, not only with narrow size distributions but also with well-defined surface chemistry and in large amounts.
In this context, a short communication was published by the group of David Schiffrin, an electrochemist from the University of Liverpool, which would set a landmark in nanoparticle research.4 The title of this paper, “Synthesis of Thiol-derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System”, summarizes very well the main achievements described in it: the small particles were obtained by a simple procedure based on a fast chemical reduction of gold ions at an oil–water interface, immediately followed by adsorption of thiolated molecules, which allowed handling of such particles as a simple chemical compound (see Fig. 1a). Additionally, the method can be easily adapted to gram-scale production.
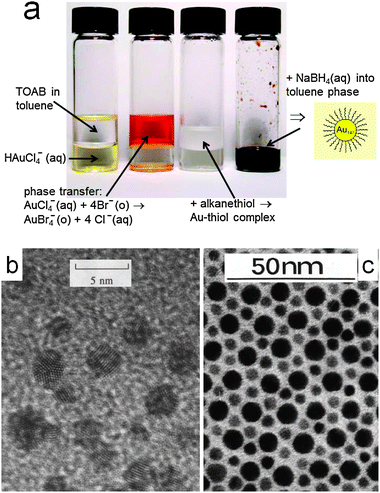 | ||
| Fig. 1 (a) Schematic view of the Brust–Schiffrin method for the synthesis of monolayer-protected clusters. (b, c) TEM images of gold nanoparticles from the original Brust et al. Chem. Commun. paper (b) and a two-dimensional binary crystal made by monodisperse gold and silver nanoparticles of appropriate sizes (c), also reported by Brust et al. Adapted with permission from ref. 10. | ||
This two-phase reduction method has triumphed over many other alternatives because of its simplicity, which gave general access to the preparation of gold nanoparticles in relatively large amounts and thus allowed the realization of a large number of experiments and additional discoveries in fields as diverse as electronics,5 catalysis,6 photochemistry,7 sensors,8 and drug delivery,9 among others. If one looks now at the transmission electron microscopy (TEM) images in the Chem. Commun. paper (Fig. 1b) for the first time, the quality of the material may seem below average in terms of polydispersity. However, it is not only remarkable that back in 1994 high resolution images were presented, but also refinement of the method was soon reported, which even allowed the first high quality binary 2D nanoparticle crystals (Fig. 1c).10
Although it should be noted that this was not the first report of thiol-functionalised gold nanoparticles (beautiful arrays of gold nanoparticles covered with alkanethiols had been earlier described by Giersig and Mulvaney11), the two-phase process largely facilitated the direct incorporation of chemical functionalities on the particles surface. This has again been a great achievement, which was further elaborated by the authors in a subsequent publication, also in Chem. Commun.12 It can be safely stated that this was the origin of an extremely prolific field that can be named “monolayer protected clusters” (the original paper mentions “surface-functionalised metallic clusters”) and which was tremendously developed by Murray and co-workers13 and used by many others. Of particular mention might be the remarkable findings of Stellacci and colleagues regarding the segregation of ligands in binary surface coatings,14 resulting in the so-called striped nanocrystals (Fig. 2), which have generated tremendous interest because of their potential to specifically place linking molecules and direct nanoparticle assembly into chains or to control the crossing of cell membranes, for example.
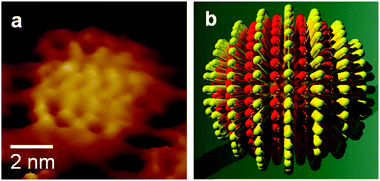 | ||
Fig. 2 STM height image (a) and corresponding schematic representation (b) of gold nanoparticles coated with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of octanethiol 1 molar ratio of octanethiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mercaptopropionic acid on Au foil. Adapted with permission from ref. 14b. mercaptopropionic acid on Au foil. Adapted with permission from ref. 14b. | ||
Despite the great success of this synthesis method, it was soon realised that it was not sufficient to focus on small metal clusters, since shape variations have a huge influence on the physical and chemical properties of metal nanoparticles. Unfortunately, the two-phase method could not be modified to obtain anisotropic nanoparticles and thus other procedures were required. The field has significantly advanced and currently a wide variety of morphologies are readily available,15 which have also allowed the demonstration of novel and extremely interesting properties. One of the fields where this has made a huge impact is nanoplasmonics, which is devoted to studying the interactions of light with such small metallic nanostructures. In this context, advances in synthetic colloid chemistry have also been key to the development of the field, including progress in theoretical methods for modelling the optical response. In 2003, Schiffrin organized a Faraday Discussions conference16 in Liverpool, which I will never forget because of the great discussions that were held during and after the sessions. I specifically remember that, although he was not heavily involved in plasmonic research himself, David recognised the importance of pushing the refinement of theoretical/numerical methods beyond Mie theory, toward a proper understanding of the experimental observations for anisotropic and complex morphologies. And he was obviously right, as demonstrated by the recent expansion of numerical methods for the resolution of Maxwell's equations applied to nanoparticles.17 This is just an additional example of the visionary spirit that was already demonstrated in the 1994 Chem. Commun. publication, where the main indications regarding the impact that the work would make have been largely confirmed. Undoubtedly, research on small metal particles is still gaining interest because of implications in a number of fundamental studies and technological applications, and we should expect many surprising discoveries that will be possible because (as Newton would say) we stand on the shoulders of giants like Brust, Schiffrin and others.
Notes and references
- L. B. Hunt, Gold Bull., 1976, 9, 134 CrossRef.
- M. Faraday, Philos. Trans. R. Soc. London, 1857, 147, 145 CrossRef.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC.
- M. Brust, D. Bethell, D. J. Schiffrin and C. Kiely, Adv. Mater., 1995, 7, 795 CrossRef CAS.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096 RSC.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834 CAS.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078 CrossRef CAS.
- R. A. Sperling, P. Rivera Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896 RSC.
- C. J. Kiely, J. Fink, M. Brust, D. Bethell and D. J. Schiffrin, Nature, 1998, 396, 444 CrossRef CAS.
- M. Giersig and P. Mulvaney, Langmuir, 1993, 9, 3408 CrossRef CAS.
- M. Brust, J. Fink, D. Bethell, D. J. Schiffrin and C. Kiely, J. Chem. Soc., Chem. Commun., 1995, 1655 RSC.
- M. J. Hostetler, A. C. Templeton and R. W. Murray, Langmuir, 1998, 15, 3782 CrossRef.
- (a) A. M. Jackson, J. W. Myerson and F. Stellacci, Nat. Mater., 2004, 3, 330 CrossRef CAS; (b) A. M. Jackson, Y. Hu, P. J. Silva and F. Stellacci, J. Am. Chem. Soc., 2006, 128, 11135 CrossRef CAS.
- M. Grzelczak, J. Pérez-Juste, P. Mulvaney and L. M. Liz-Marzán, Chem. Soc. Rev., 2008, 37, 1783 RSC.
- Faraday Discuss, 2004, 125, 1–414 Search PubMed.
- V. Myroshnychenko, J. Rodríguez-Fernández, I. Pastoriza-Santos, A. M. Funston, C. Novo, P. Mulvaney, L. M. Liz-Marzán and F. J. García de Abajo, Chem. Soc. Rev., 2008, 37, 1792 RSC.
| This journal is © The Royal Society of Chemistry 2013 |
