Global and local reactivity indices for electrophilic/nucleophilic free radicals†
Abstract
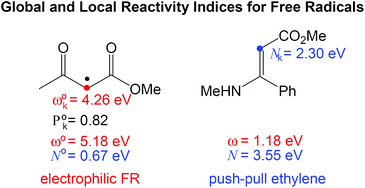
A set of five DFT reactivity indices, namely, the global electrophilicity ω° and nucleophilicity N° indices, the radical Parr function P°k, and the local electrophilicity ω°k and nucleophilicity N°k indices, for the study of free radicals (FRs) are proposed. Global indices have been tested for a series of 32 FRs having electrophilic and/or nucleophilic activations. As expected, no correlation between the proposed global electrophilicity ω° and global nucleophilicity N° has been found. Analysis of the local electrophilicity ω°k and nucleophilicity N°k indices for FRs, together with analysis of the local electrophilicity ωk and nucleophilicity Nk indices for

 Please wait while we load your content...
Please wait while we load your content...