 Open Access Article
Open Access ArticlePostulates on electromagnetic activity in biological systems and cancer
Jiří
Pokorný
a,
Jan
Pokorný
*bc and
Jitka
Kobilková
d
aInstitute of Photonics and Electronics, Academy of Sciences of the Czech Republic, Prague, Czech Republic. E-mail: pokorny@ufe.cz
bInstitute of Physics, Academy of Sciences of the Czech Republic, Prague, Czech Republic. E-mail: pokorny@fzu.cz
cDepartment of Materials Science and Engineering, The University of Sheffield, UK
dDepartment of Gynecology and Obstetrics, Ist Medical Faculty of Charles University, Prague, Czech Republic
First published on 14th October 2013
Abstract
A framework of postulates is formulated to define the existence, nature, and function of a coherent state far from thermodynamic equilibrium in biological systems as an essential condition for the existence of life. This state is excited and sustained by energy supply. Mitochondria producing small packets of energy in the form of adenosine and guanosine triphosphate and strong static electric field around them form boundary elements between biochemical–genetic and physical processes. The transformation mechanism of chemical energy into useful work for biological needs and the excitation of the coherent state far from thermodynamic equilibrium are fundamental problems. The exceptional electrical polarity of biological objects and long-range interactions suggest a basic role of the endogenous electromagnetic field generated by living cells. The formulated postulates encompass generation, properties and function of the electromagnetic field connected with biological activity and its pathological deviations. Excited longitudinal polar oscillations in microtubules in eukaryotic cells generate the endogenous electromagnetic field. The metabolic activity of mitochondria connected with water ordering forms conditions for excitation. The electrodynamic field plays an important role in the establishment of coherence, directional transport, organization of morphological structures, interactions, information transfer, and brain activity. An overview of experimental results and physical models supporting the postulates is included. The existence of the endogenous biological electromagnetic field, its generation by microtubules and supporting effects produced by mitochondria have a reasonable experimental foundation. Cancer transformation is a pathological reduction of the coherent energy state far from thermodynamic equilibrium. Malignancy, i.e. local invasion and metastasis, is a direct consequence of mitochondrial dysfunction, disturbed microtubule polar oscillations and the generated electromagnetic field.
Insight, innovation, integrationLiving organisms are driven by continuous energy supply. Although energy is the main topic in physics, energetic characterization of processes in living matter has been neglected to a large extent. The authors summarize a nearly 50-year history of forming a new interdisciplinary field, present latest experimental and theoretical achievements including a comprehensive functional description of energetic processes in biology, and chart a course for detailed research in future. The framework of postulates makes possible integration of energy dependent processes into classical biology based on chemical–genetic principles. This article shows that still an unknown part of cancer transformation depends on physical energetic mechanisms. |
Introduction
The laws of physics governing living organisms are not adequately investigated. Despite massive support of interdisciplinary research during the last couple of years, numerous studies of physical aspects are still being limited to simulating marginal effects without addressing fundamental mechanisms in living matter.1 In analogy to quantum mechanics, we formulate a framework of postulates encompassing the fundamentals of biological activity. The postulates predominantly deal with energy generation and conversion, the fundamental issue in physics in general. Some of the postulates already have reasonable experimental support.Progress in biochemical–genetic research has created the conditions for studying and understanding of physical processes in biology. A living organism is a complex system composed of a great amount of different, non-linearly interacting entities organized in a hierarchical structure.2 Such a complex system is far from thermodynamic equilibrium, dissipative, and self-organized. Adaptability to surrounding conditions and stimuli is an outstanding active feature. All living systems display quite an extraordinary property: a certain level of awareness of their difference from the surroundings, their existence. Consciousness and connected instinct of self-preservation are highly evolved in mammals.
The state far from thermodynamic equilibrium is built by energy supply. Energy transformation and utilization in biological systems is a hallmark of life. Energy supply makes transport, interactions, organization and information transfer possible. Any motion depends on energy supply. Processes based on energy supply are essential for biological activity.
A living organism transforms chemical energy into a form suitable for entering biological processes. Description of the transformation mechanism is a principal task. Chemical bonds (including covalent, ionic, hydrogen, and van der Waals type) were commonly assumed to be dominating for biological organization and activity. However, these bonds represent the forces of a short-range nature in the nm region. Biological systems maintain order at every level of dimensions and display even a macroscopic long-range order and interactions. For instance, order is strikingly apparent in large structures such as a butterfly wing.3 The biological order is discussed in general terms of energy supply and the release of heat from cells. Order may be explained by long-range forces of physical origin and adequate high capacity of information transfer. The exceptional electrical polarity of biological objects suggests a fundamental role of endogenous electromagnetic fields in biological activity. Since the majority of proteins and protein structures are electrically polar,4–8 any oscillation generates electromagnetic field. Therefore, biological activity should be based not only on biochemical–genetic processes but also on biophysical mechanisms of the electromagnetic field. Research on electromagnetic activity of biological systems was initiated by a hypothesis presented by H. Fröhlich at the First Versailles Conference on Theoretical Physics and Biology in 1967.9 Fröhlich assumed strong excitation of one or a few modes of motion, stabilized due to low emission and friction losses, phases correlated over macroscopic regions, and superimposed on random thermal fluctuations. He recognized that the strong polarity of biological objects suggests longitudinal electric oscillations as stabilizing modes.9 Fröhlich's hypothesis laid the basis for understanding physical processes in biological systems. Postulates concerning generation and function of the electromagnetic field in biological systems and cancer development are presented in the next part. The postulates set up a solid foundation for further theoretical and experimental studies in a new interdisciplinary field.
Postulate I
Eukaryotic living cells generate an endogenous electromagnetic field; the field is coherent
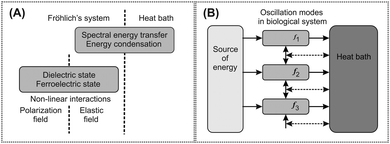 | ||
| Fig. 1 An abstract physical model of Fröhlich's hypothesis for generation of an electromagnetic field in biological systems. A schematic picture of the generation process includes mechanisms based on longitudinal elastic and polarization oscillations, and energy transfer from the source to the oscillation systems and from them to the heat bath. The term mode is used for oscillation at a distinct frequency and energy equivalent with a harmonic oscillator. The source and the heat bath are any parts of the model system supplying and accepting energy from the oscillating modes, respectively. (A) Non-linear interaction between the polarization field and the longitudinal elastic field and spectral energy transfer between oscillating modes along the frequency scale are the essential mechanisms. (B) Energy is supplied from the source to particular modes with different frequencies. Energy flow from the modes to the heat bath causes losses (horizontal solid arrows). Due to non-linear coupling between the modes with different frequencies and the heat bath, energy is transferred between modes (the transfers are denoted by vertical solid arrows and horizontal dashed arrows). Energy may be transferred between any two modes but only transfer between neighbouring modes is denoted. Energy condenses in the lowest frequency mode. | ||
Measurements performed on living cells disclosed electric and electromagnetic oscillations. Dielectrophoretic attraction of dielectric particles to living cells was observed and the corresponding frequency of oscillations was assessed in the range below 10 MHz by Pohl et al.12 Besides dielectrophoretic measurements of yeast and alga cells, Hölzel13 measured oscillations in the frequency range 1.5–52 MHz using a special detection and amplification system. The external electromagnetic field is damped by cancer tissue at the frequency of 465 MHz and its first harmonic.14 Electromagnetic field generated by living cells in the red and near-infrared regions causes an interaction between them.15 Elongated BHK cells on opposite faces of thin and thick glass films were oriented in transverse and random directions, respectively. A metal layer (deposited on the glass film) absorbing the electromagnetic field disturbed the orientation effect. The orientation effect was preserved after deposition of a silicon layer strongly absorbing the blue end of the visible spectrum. Cells also detect electromagnetic signals and send pseudopodia to the source of light.16 Photon emission from living bodies was measured for instance by Popp.17 Experimental results suggest that eukaryotic living cells can generate an electromagnetic field in a wide frequency spectrum.
Postulate II
Microtubules are non-linear oscillating structures generating an electromagnetic field in living cells
The structure generating the field has not been known. Fröhlich11 assumed generating structures in the plasma membrane. Microtubule filaments, hollow tubes with inner and outer diameters of 17 and 25 nm, respectively, were described by Amos and Klug.18 Microtubule radial structure in the cell is the main organizer of the cytoskeleton. Experimental research on the cellular electromagnetic activity gradually pointed to microtubules as generating structures of the cellular electromagnetic field. Large dielectrophoretic effects of the yeast cells in the M phase (when cells divide and microtubule activity is high) were measured by Pohl et al.12 Hameroff19 suggested Fröhlich-type coherent excitation in the cytoskeleton. Tuszyński et al.20 proved that heterodimers in microtubules are electric dipoles. The generation of electromagnetic field by microtubules based on Fröhlich's mechanism of electric polar vibrations was proposed by Pokorný et al.21 In subsequent years this idea was supported by a measurement of electric oscillations at the plasma membrane of living cells in the M phase which displays enhanced electric activity in some periods coinciding with mitotic spindle formation and its specific developmental features.22 Microtubule electromagnetic activity in heterodimer attachment was also suggested to occur by disruption of microtubule polymerization in cells by an external electromagnetic field at the frequency of 0.1–0.3 MHz.23 Resonant frequencies of microtubules in the frequency range of 10–30 MHz and 100–200 MHz were reported by Sahu et al.24 Their research and its outstanding results deserve at least a short description. The resonant frequencies were obtained by measurement of dc conductivity after application of an oscillating electromagnetic signal and from transmittance and reflectance of microtubules with and without compensation of parasitic reactance in the frequency range of 1 kHz–1.3 GHz. These experiments showed that the resonant frequencies do not depend on the length of the microtubule. The resonant peaks were not observed after release of water from the microtubule cavity.
The physical mechanisms of oscillations in microtubules generating an electromagnetic field in the frequency bandwidth below about 1 THz are not completely understood. Fröhlich suggested longitudinal polar modes.9–11 Measurements by Sahu et al.24 on microtubules disclosed electric resonances and their special properties and conditions of excitation. Free electric charges in ordered water participate in oscillations and provide cooperation between tubulin heterodimers. All tubulin heterodimers oscillate at the same frequencies. Frequencies measured on the microtubule correspond to those of individual tubulin heterodimers. The frequencies depend on the modulus of elasticity of vibrations and/or on the secondary structure of the tubulin heterodimer. In addition, the effect of ordered water in the microtubule cavity on oscillations might be substantial.
Energy for microtubule excitation is supplied by several mechanisms: by hydrolysis of GTP to GDP in β tubulins after polymerization,21,25 motion of motor proteins along microtubules,26 and very likely also by non-utilized energy liberated from mitochondria.27,28 Chemical reactions release photons in the UV and visible part of the wavelength spectrum and may in this way supply energy to oscillations too. Apart from an active source, some parts of biological systems may represent passive resonant circuits. It is assumed that a living cell forms a cavity resonator for electromagnetic waves at the frequency of about 1013 Hz, which corresponds to a cell of e.g. a spherical shape with a diameter about 10 μm.29 The positions of mitotic spindle poles may correspond to the nodes of the cavity electromagnetic field and the geometrical shape of polar and kinetochore microtubules to the line of force of the field.17 Microtubular inner dimensions correspond to a soft X-ray cavity resonator.29
Postulate III
Mitochondria establish conditions for generation of the electromagnetic field
Before describing the additional mitochondrial effect a periodic character of the cell development in time should be mentioned. The cell cycle has distinct phases. The M phase denotes the process of nuclear division and separation into two cells. The remaining portions of the cell cycle are included in the interphase, i.e. the period between two M phases. In the interphase mitochondria are aligned along microtubules in the regions of greatest energy consumption. The strong static electric field around mitochondria may shift oscillations in molecules and structures into a highly non-linear region.31,36 Water viscosity damping of microtubule oscillations is significantly reduced due to ordered water around them.36 The first experimental proof of a special clear 5–20 nm thick layer around microtubules (later explained as an ordered water layer) was published by Amos37 and analysis of the effect of a protective layer on damping was done by Pokorný.38
Cooperation of mitochondria and microtubules shown in Fig. 2 is a unique property for biological functions. Mitochondria are the boundary units between biochemical–genetic and physical processes.
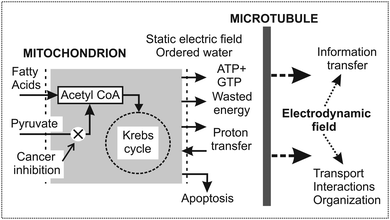 | ||
| Fig. 2 Function of mitochondria and microtubules in excitation of microtubular oscillations and generation of the electromagnetic field in cells. Mitochondria are at the boundary between chemical–genetic and physical processes in cells. Mitochondria provide energy, water ordering, and a strong static electric field. In cancers the mitochondrial dysfunction is created either in cancer cells (the Warburg effect) or fibroblasts associated with cancer cells (the reverse Warburg effect – RWE). In the former case pyruvate transfer into mitochondria in cancer cells is inhibited (denoted by a cross) and the general shift of biological activity is downwards.56 In the latter case a large amount of energy rich metabolites is transported to cancer cells from the associated fibroblasts. Due to enhanced biological activity, RWE cancer cells are highly aggressive.59 (Published with a kind permission of the journal Prague Medical Report57). | ||
Postulate IV
Synchronization, force effects for transport, interactions, morphology, and information transfer are functions of the electromagnetic field
The essential transporting function inside a cell is provided by motor proteins moving along microtubules. Directional transport of biological molecules and reaction components in the cytosol is difficult to be explained purely by Brownian motion. Combination of electrodynamic deterministic and random forces in directional transport of mass particles was analysed.42 The organization of living matter might depend on directional transport43 and the pattern formation field (morphogenetic field) which determines the morphological structure. Communication between the brain and various parts of the body could be mediated by streams of photons, which may provide a high-capacity information transfer.44
Postulate V
Consciousness, instinct of self-preservation, and the central control function of the brain and less developed structures depend on quantum electrodynamic processes
R. Penrose formulated a novel hypothesis of wave function self-collapse (objective reduction), i.e. how quantum superimposed states are reduced to a single, classical state.49 A coherent quantum system can self-collapse if by growing and persisting it reaches the threshold of a critical quantity (mass, time, energy). R. Penrose and S. Hameroff applied the hypothesis of orchestrated reduction of quantum coherence to brain function based on microtubules. The reduction outcomes need not be random, but can reflect a result of computation occurring in the coherent superposition state. The idea of objective reduction is assumed to be essential for consciousness. At present mainly theoretical analyses are published.50 However, further experimental data on microtubules as memory elements and processing systems would disclose the main concepts of the brain function.
Postulate VI
Disturbances of the energy processing system and the coherent electrodynamic state far from thermodynamic equilibrium produce pathological states, in particular cancer
In glycolytic phenotype cancer cells, fermentation provides about one half of the cellular ATP production (aerobic glycolysis).53,54 In healthy cells the oxidative ATP production may be even 100 times greater than the fermentative one. Measurement of cancer cells performed by Damadian58 displayed a less ordered system. Viscosity properties of water at low levels of ordering causing damping of oscillations in microtubules and diminished static electric fields might result in a shift of oscillations toward the linear region.36 Consequently, the power of the electrodynamic field is lowered, coherence diminished, and the frequency spectrum shifted and rebuilt.
Nevertheless, another picture of the cancer process was observed by Pavlides et al.59 Epithelial breast cancer cells have fully functional mitochondria, and energy rich metabolites (pyruvate, lactate, etc.) produced by fermentation are supplied to them from the associated fibroblasts with dysfunctional mitochondria.59 (Fibroblasts are cells secreting protein fibres forming connective tissue filling the space between organs and tissues in the body. The connective tissue underlying an epithelial cell sheet is termed stroma.) Cancer cells take up these metabolites and use them in the mitochondria, thereby promoting efficient energy production.59 Increased ATP generation via oxidative phosphorylation depends on increased proton transfer from the matrix space which increases the static electric field around mitochondria. Data presented by Pavlides et al.59 reveal that the power of the electrodynamic field is high and as a result the microtubule oscillations are shifted to a highly non-linear region, and the frequency spectrum is changed. The energy-rich supply from the environment supports cancer cell growth and its aggressiveness. The described type of cancers studied by Pavlides et al.59 is connected with the term “the reverse Warburg effect”.
Power and suggested frequency shifts of the two mechanisms of cancers (i.e. with the Warburg and reverse Warburg effects) are depicted in Fig. 3. Cancer tissues display an absorption resonance frequency at 465 MHz.36 Resonance spectra of microtubules from healthy cells are below about 200 MHz.24
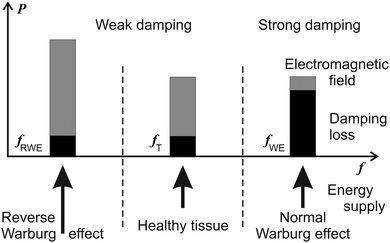 | ||
| Fig. 3 Spectral position of the power P which may be transformed from the microtubule oscillations and the generated electromagnetic field into useful work (the grey parts) or lost by damping of microtubule oscillations (the black parts). A schematic picture of the hypothetical dependence of the power P on frequency f in a healthy cell, cancer cells with the Warburg and the reverse Warburg effect. The microtubule is assumed to be a non-linear oscillator. Cancer cells with the Warburg effect exhibit high damping of oscillations. | ||
The function of biological organs depends on enslavement of the cells in the tissue based on mutual interactions between cells. A model theoretical analysis based on a physical electromagnetic mechanism can explain interactions between cells in the tissue.31,36,60 The interaction depends on the frequency spectrum, the space pattern, and the power of the electromagnetic field generated by the interacting entities (the cells). The cancer cell escapes from interactions with the surrounding healthy cells and performs individual activity if its frequency spectrum is rebuilt and shifted39 and/or the space pattern disturbed. The space pattern is determined by geometrical arrangement of the sources (microtubules) and their surrounding parts (other cytoskeleton structures). The model requirements are compared with the real cellular properties. Human non-tumorigenic epithelial breast cells have smaller deformability than cells with increased metastatic potential – 10 and 30%, respectively.61 Bioactive lipid sphingosylphosphorylcholine (SPC) that influences cancer metastasis causes shrinking of the keratin network around the nucleus62 and consequently may cause diminished interactions based on microtubule oscillations and electromagnetic fields. Interaction forces between cancer cells may differ from those between healthy cells or healthy and cancer cells.39–41 The force effect together with disturbances of the intercellular matrix may constitute an essential part of the local invasion and metastasis. This process is well described and referred to as the epithelial-to-mesenchymal transition.
Mitochondria in glycolytic phenotype cancer cells display a so-called “hyperpolarized” inner membrane that is always associated with low expression of K+ channels in the plasma membrane and increased resistance to apoptosis.56 However, uptake and retention of fluorescent dyes used for measurement of mitochondrial membrane potential may depend also on distribution of ions in the cell63 (for instance K+), production of lactate, and a layer of ordered water. This type of cancer cells was found in many types of cancers, for instance in a great majority of adenocarcinomas, carcinomas, and melanomas.63,64 The lack of mitochondrial “hyperpolarization” in certain types of cancer cells suggests a modified glycolytic phenotype, which may correspond to the reverse Warburg effect. This type of cancer cells was observed in lymphomas, sarcomas, neuroblastomas,63,64 breast cancers, advanced prostatic cancers, and very likely exists in many different types of epithelial cancers dependent on the tumour microenvironment.59
The diversity of cancer origin agents also led to a hypothesis that mitochondrial dysfunction is a primary cause of cancer, and biochemical and genetic deviations develop as a consequent event.65 This hypothesis contradicts the experimental results connecting mitochondrial dysfunction with chemical–genetic defects. On the other hand dysfunction of mitochondria might be caused by a specific agent directly with no previous alterations of chemical and genetic processes. Lactate dehydrogenase virus (LDV) parasiting on an energy system is observed as a dark particle at mitochondria.55 The possible role of LDV in cancer origin, course, and progress is not clear.
Disturbances of biophysical processes might be caused also by defects in the link of generation of the microtubule oscillations and the electromagnetic field. A hypothesis explains asbestos carcinogenicity by the capability of the asbestos fibres to short-circuit distant parts of the cell with different levels of the electromagnetic field. Asbestos by itself may form optic fibres for the cellular field66 and/or conductive wires after adsorption of specific proteins and molecules containing iron atoms at the fibres surface forming conductive layers.67 Nevertheless, other hypotheses explain asbestos carcinogenicity on the basis of the mechanism of oxidative stress and chromosome tangling.67 A hypothetical overview of the cancer transformation pathway is shown in Table 1.
| The pathway is divided into three parts: (a) the initial phase – chemical and genetic links, (b) the intermediate phase – mitochondrial dysfunction and enhanced glycolysis in cancer cells or fibroblasts associated with cancer cells, (c) the final phase – the rebuilt and shifted frequency spectrum. Traill's hypothesis66 shifts the beginning of asbestos cancer beyond mitochondrial dysfunction in the region of defects of microtubular oscillations and electromagnetic field. Release from the tissue regulation due to changed frequency spectrum enables the cell to act independently.39 |
|---|

|
Conclusions
The postulates formulated in the text are based on the present human knowledge of biological activity. The first three postulates concerning the endogenous coherent electromagnetic field generated by oscillations in microtubules under conditions established by mitochondria have a strong experimental support. Experimental data on living cells indicate the presence of an electromagnetic field in a wide frequency spectrum. Accurate specification of the parameters of the field, in particular the frequency spectrum, coherence, power, space pattern and their alterations dependent on biological conditions and pathological states have to be measured.The role of microtubule oscillations and electromagnetic field in biological functions such as transport, interactions, information transfer, and quantum processes in the brain etc. is mainly based on theoretical predictions but partial experimental results support the ideas. The brain might be assumed to correspond to a system performing analog–digital processing.
Physical links in cancer transformation are in the realm behind the Warburg effect. Cancer research and treatment should be targeted to this area and current achievements are opening the path to do so.
Physical processes can be observed as a consequence of their effects on material objects. Energy, force, and velocity of motion are among the most important physical quantities. Knowledge of the living bodies, cells and subcellular structures should be expanded by characterizing the fundamental physical processes. The postulates form a framework for integral description of biological mechanisms and development of adequate experimental techniques.
Acknowledgements
This study was supported by grant No. P102/11/0649 of the Czech Science Foundation GA CR.Notes and references
- F. Michor, J. Liphardt, M. Ferrari and J. Widom, Nat. Rev. Cancer, 2011, 11, 657–670 CrossRef CAS PubMed.
- J. Kwapień and S. Droždž, Phys. Rep., 2012, 15, 115–226 CrossRef PubMed.
- B. Alberts, D. Bray, J. Lewis, M. Raff, K. Roberts and J. D. Watson, Molecular Biology of the Cell, Garland Publishing, New York & London, 1994 Search PubMed.
- A. Wada, Adv. Biophys., 1976, 9, 1–63 CAS.
- A. Wada and H. Nakamura, Nature, 1981, 293, 757–758 CrossRef CAS.
- H. Nakamura and A. Wada, J. Phys. Soc. Jpn., 1985, 54, 4042–4046 CrossRef.
- H. Nakamura and A. Wada, J. Phys. Soc. Jpn., 1985, 54, 4047–4052 CrossRef CAS.
- N. A. Baker, D. Sept, S. Joseph, M. J. Holst and J. A. McCammon, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 10037–10041 CrossRef CAS PubMed.
- H. Fröhlich, in Theoretical Physics and Biology, ed. M. Marois, North-Holland, Amsterdam, 1969, pp. 13–22. Proc. 1st Int. Conf. Theor. Phys. Biol., Versailles, 1967 Search PubMed.
- H. Fröhlich, Collect. Phenom., 1973, 1, 101–109 Search PubMed.
- H. Fröhlich, Adv. Electron. Electron Phys., 1980, 53, 85–152 CrossRef.
- H. A. Pohl, T. Braden, S. Robinson, J. Piclardi and D. G. Pohl, J. Biol. Phys., 1981, 9, 133 CrossRef.
- R. Hölzel, Electro- Magnetobiol., 2001, 20, 1–13 Search PubMed.
- C. Vedruccio and A. Meessen, in Proceedings PIERS, Progress in Electromagnetics Research Symposium, Pisa, Italy, March 28–31, 2004, pp. 909–912.
- G. Albrecht-Buehler, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 8288–8293 CrossRef CAS.
- G. Albrecht-Buehler, J. Cell Biol., 1991, 114, 493–502 CrossRef CAS.
- F.-A. Popp, in Herbert Fröhlich, FRS. A Physicist Ahead of his Time, ed. G. J. Hyland and P. Rowlands, The University of Liverpool, Liverpool, 2006, pp. 155–192 Search PubMed.
- L. A. Amos and A. Klug, J. Cell Sci., 1974, 14, 523–549 CAS.
- S. R. Hameroff, in Biological Coherence and Response to External Stimuli, ed. H. Fröhlich, Springer-Verlag, Berlin, Heidelberg, New York, 1988, pp. 242–265 Search PubMed.
- J. A. Tuszyński, S. Hameroff, M. Satarić, B. Trpisová and M. L. A. Nip, J. Theor. Biol., 1995, 174, 371–380 CrossRef.
- J. Pokorný, F. Jelínek, V. Trkal, I. Lamprecht and R. Hölzel, J. Biol. Phys., 1997, 23, 171–179 CrossRef.
- J. Pokorný, J. Hašek, F. Jelínek, J. Šaroch and B. Palán, Electro- Magnetobiol., 2001, 20, 371–376 Search PubMed.
- E. D. Kirson, Z. Gurvich, R. Schneiderman, E. Dekel, A. Itzhaki, Y. Wasserman, R. Schatzberger and Y. Palti, Cancer Res., 2004, 64, 3288–3295 CrossRef CAS.
- S. Sahu, G. Subrata, B. Ghosh, K. Aswani, K. Hirata, D. Fujita and A. Bandyopadhyay, Biosens. Bioelectron., 2013, 47, 141–148 CrossRef CAS PubMed.
- J. Pokorný, Bioelectrochem, 2004, 63, 321 CrossRef PubMed.
- A. E. Pelling, S. Sehati, E. B. Gralla, J. S. Valentine and J. K. Gimzewski, Science, 2004, 305, 1147–1150 CrossRef CAS PubMed.
- J. Pokorný, J. Hašek, J. Vaniš and F. Jelínek, Indian J. Exp. Biol., 2008, 46, 310–321 Search PubMed.
- J. Pokorný, Electromagn. Biol. Med., 2009, 28, 105–123 CrossRef PubMed.
- F. Jelínek and J. Pokorný, Electro- MagnetoBiol., 2001, 20, 75–80 Search PubMed.
- K. M. Tyner, R. Kopelman and M. A. Philbert, Biophys. J., 2007, 93, 1163–1174 CrossRef CAS PubMed.
- J. Pokorný, AIP Adv., 2012, 2, 011207 CrossRef.
- J. Zheng, W. Chin, E. Khijniak and G. H. Pollack, Adv. Colloid Interface Sci., 2006, 127, 19–27 CrossRef CAS PubMed.
- E. C. Fuchs, J. Woisetschlager, K. Gatterer, E. Maier, R. Pecnik, G. Holler and H. Eisenkolbl, J. Phys. D: Appl. Phys., 2007, 40, 6112–6114 CrossRef CAS.
- G. Preparata, QED Coherence in Matter, World Scientific, Hong Kong, New Jersey, London, 1995 Search PubMed.
- E. Del Giudice and A. Tedeschi, Electromagn. Biol. Med., 2009, 28, 46–52 CrossRef PubMed.
- J. Pokorný, C. Vedruccio, M. Cifra and O. Kučera, Eur. Biophys. J., 2011, 40, 747–759 CrossRef PubMed.
- L. A. Amos, in Microtubules, ed. K. Roberts and J. S. Hyam, Academic Press, London, New York, 1979, pp. 1–64 Search PubMed.
- J. Pokorný, Electromagn. Biol. Med., 2003, 22, 15–29 CrossRef PubMed.
- H. Fröhlich, IEEE Trans. Microwave Theory Tech., 1978, 26, 613–617 CrossRef.
- J. Pokorný and T.-M. Wu, Biophysical Aspects of Coherence and Biological Order, Academia, Prague, Springer-Verlag, Berlin, Heidelberg, New York, 1998 Search PubMed.
- J. Pokorný, in Herbert Fröhlich, FRS. A Physicist Ahead of his Time, ed. G. J. Hyland and P. Rowlands, The University of Liverpool, Liverpool, 2006, pp. 193–224 Search PubMed.
- J. Pokorný, Electro- Magnetobiol., 2001, 20, 59–73 Search PubMed.
- J. Pokorný, J. Hašek and F. Jelínek, Electromagn. Biol. Med., 2005, 24, 185–197 CrossRef.
- J. Pokorný, T. Martan and A. Foletti, J. Acupunct. Meridian Stud., 2012, 5, 34–41 CrossRef PubMed.
- K. M. Yamada, B. S. Spooner and N. K. Wessells, Proc. Natl. Acad. Sci. U. S. A., 1970, 66, 1206–1212 CrossRef CAS.
- L. Dehmelt and S. Halpain, J. Neurobiol., 2004, 58, 18–33 CrossRef CAS PubMed.
- A. Priel, A. R. Ramos, J. A. Tuszyński and H. F. Cantiello, Biophys. J., 2006, 90, 4639–4643 CrossRef CAS PubMed.
- S. Sahu, S. Ghosh, K. Hirata, D. Fujita and A. Bandyopadhyay, Appl. Phys. Lett., 2013, 102, 123701 CrossRef.
- R. Penrose, Shadows of the Mind, Oxford Press, London, 1994 Search PubMed.
- S. Hagan, S. Hameroff and J. Tuszyński, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2002, 65, 061901 CrossRef CAS.
- T. M. Devlin, Textbook of Biochemistry with Clinical Correlations, Wiley, Hoboken, NJ, 7th edn, 2011 Search PubMed.
- A. Jandová, K. Motyčka, J. Čoupek, J. Sanitrák, K. Heyberger, L. Laurová, B. Mejsnarová, J. Novotná, J. Pezlarová, F. Macholda, J. Šimonová, C. Dostál and Z. Kubát, Sb. Lék., 1979, 81, 321–327 Search PubMed.
- O. Warburg, K. Posener and E. Negelein, Biochem. Z., 1924, 152, 309–344 CAS.
- O. Warburg, Science, 1956, 123, 309 CAS.
- A. Jandová, J. Pokorný, J. Kobilková, M. Janoušek, J. Mašata, S. Trojan, M. Nedbalová, A. Dohnalová, A. Beková, V. Slavík, A. Čoček and J. Sanitrák, Electromagn. Biol. Med., 2009, 28, 1–14 CrossRef PubMed.
- S. Bonnet, S. L. Archer, J. Allalunis-Turner, A. Haromy, Ch. Beaulieu, R. Thompson, Ch. T. Lee, G. D. Lopaschuk, L. Puttagunta, S. Bonnet, G. Harry, K. Hashimoto, Ch. J. Porter, M. A. Andrade, B. Thebaud and E. D. Michelakis, Cancer Cell, 2007, 11, 37–51 CrossRef CAS PubMed.
- J. Pokorný, A. Jandová, M. Nedbalová, F. Jelínek, M. Cifra, O. Kučera, D. Havelka, J. Vrba, J. Vrba Jr, A. Čoček and J. Kobilková, Prague Med. Rep., 2012, 113, 81–94 Search PubMed.
- R. Damadian, Science, 1971, 171, 1151–1153 CAS.
- S. Pavlides, D. Whitaker-Menezes, R. Castello-Cros, N. Flomenberg, A. K. Witkiewicz, P. G. Frank, M. C. Casimiro, Ch. Wang, P. Fortina, S. Addya, R. G. Pestell, U. E. Martinez-Outschoorn, F. Sotgia and M. P. Lisanti, Cell Cycle, 2009, 8, 3984–4001 CrossRef CAS.
- J. Pokorný, A. Foletti, J. Kobilková, A. Jandová, J. Vrba, J. Vrba Jr, M. Nedbalová, A. Čoček, A. Danani and J. A. Tuszyński, Sci. World J., 2013, 2013, 195028 CrossRef PubMed.
- J. Guck, S. Schinkinger, B. Lincoln, F. Wottawah, S. Ebert, M. Romeyke, D. Lenz, H. M. Erickson, R. Ananthakrishnan, D. Mitchell, J. Käs, S. Ulvick and C. Bilbi, Biophys. J., 2005, 88, 3689–3698 CrossRef CAS PubMed.
- M. Beil, A. Micoulet, G. von Wichert, S. Paschke, P. Walther, M. B. Omary, P. P. Van Veldhoven, U. Gern, E. Wolff-Hieber, J. Eggermann, J. Waltenberger, G. Adler, J. Spatz and T. Seufferlein, Nat. Cell Biol., 2003, 5, 803–811 CrossRef CAS PubMed.
- L. B. Chen, Annu. Rev. Cell Biol., 1988, 4, 155–181 CrossRef CAS PubMed.
- E. D. Michelakis, L. Webster and J. R. Mackey, Br. J. Cancer, 2008, 99, 989–994 CrossRef CAS PubMed.
- T. N. Seyfried and L. M. Shelton, Nutr. Metab., 2010, 7, 7 Search PubMed.
- R. R. Traill, in 9th Int. Fröhlich's Symposium, J. Phys. 2011: Conference Series 329, 012017.
- S. Toyokuni, Nagoya J. Med. Sci., 2009, 71, 1–10 CAS.
| This journal is © The Royal Society of Chemistry 2013 |
