BODIPY dyes in photodynamic therapy
Anyanee
Kamkaew
a,
Siang Hui
Lim
bc,
Hong Boon
Lee
b,
Lik Voon
Kiew
d,
Lip Yong
Chung
c and
Kevin
Burgess
*a
aDepartment of Chemistry, Box 30012, Texas A & M University, College Station, TX 77841-3012, USA. E-mail: burgess@tamu.edu
bCancer Research Initiatives Foundation (CARIF), Subang Jaya Medical Centre, 47500 Subang Jaya, Selangor, Malaysia
cDepartment of Pharmacy, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia
dDepartment of Pharmacology, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia
First published on 26th September 2012
Abstract
BODIPY dyes tend to be highly fluorescent, but their emissions can be attenuated by adding substituents with appropriate oxidation potentials. Substituents like these have electrons to feed into photoexcited BODIPYs, quenching their fluorescence, thereby generating relatively long-lived triplet states. Singlet oxygen is formed when these triplet states interact with 3O2. In tissues, this causes cell damage in regions that are illuminated, and this is the basis of photodynamic therapy (PDT). The PDT agents that are currently approved for clinical use do not feature BODIPYs, but there are many reasons to believe that this situation will change. This review summarizes the attributes of BODIPY dyes for PDT, and in some related areas.
 Anyanee Kamkaew | Anyanee Kamkaew received her BSc in Chemistry from Silpakorn University in Nakorn Pathom, Thailand, in 2007. She received a Development and Promotion of Science and Technology Talents Project (DPST) scholarship from Royal Government of Thailand in 2009 to pursue her PhD under the guidance of Prof. Kevin Burgess at Texas A&M University. Her current research of interest is in the field of organic and biomolecular synthesis to probe intramolecular delivery and targeting. |
 Siang Hui Lim | Siang Hui Lim received his BSc in Biomedical Sciences from University Kebangsaan Malaysia in Kuala Lumpur, then did his MSc research work in the field of Molecular Medicine in University Putra Malaysia in Selangor. Since 2007, he served as a Research Scientist in a non-profit cancer research institution in Malaysia, Cancer Research Initiatives Foundation (CARIF). His current research focuses on characterizing the photodynamic activity of potential photosensitizers and the antitumor activity of potential antineoplastic agents. He is currently pursuing his PhD in the field of photodynamic therapy focusing on improving the delivery of photosensitizers. |
 Hong Boon Lee | Hong Boon Lee obtained her BA (1996) and PhD (2000) in Organic Chemistry from the University of Cambridge, UK. Her PhD thesis under the tutelage of Prof. Shankar Subramanian, Department of Chemistry, focused on new methodologies in solid-phase synthesis of small molecules. She then won a post-doctoral research fellowship from the Wellcome Trust, UK, to make bioactive peptidomimetics under the guidance of Prof. Kevin Burgess at Texas A&M University, USA. Since 2002, Dr Lee has been conducting research at CARIF, Malaysia, where she combines chemistry and biology to discover and develop new anti-cancer compounds including photosensitizers for photodynamic therapy. |
 Lik Voon Kiew | Lik Voon Kiew received his doctorate from the University of Malaya, Malaysia, in 2008. He is currently a senior lecturer at the Pharmacology department, Faculty of Medicine of the University of Malaya, Malaysia. His current research interests include the development and in vitro/in vivo evaluation of cancer targeting drug carriers and anticancer photosensitizers. |
 Lip Yong Chung | Lip Yong Chung received his doctorate in pharmacy from the University of Cardiff, UK, in 1990. He joined Cardiff University as a research associate focusing on industry sponsored research and is currently a Professor in Pharmaceutical Sciences at the Faculty of Medicine of the University of Malaya, Malaysia. His current research interests include the design of bioactive molecules of pharmacological interest and the study of targeting biological systems. |
 Kevin Burgess | Suspecting that medicinal chemists have historically over-emphasised cytotoxicity data and under-emphasised the importance of chemical methods to target tumors, Kevin Burgess has become interested in the idea of using two targeting techniques simultaneously. Photodynamic therapy (PDT) is spatially targeted (to areas that are irradiated), and could be coupled with molecular fragments for active targeting of tumors. Burgess with co-workers at TAMU and in KL wrote this review to obtain a better understanding of what, if anything, had been done with BODIPY PDT agents in this regard. |
Introduction
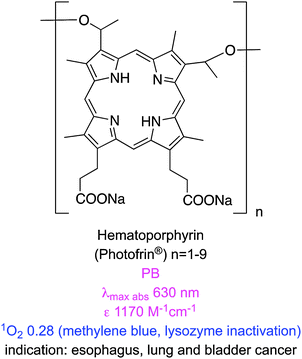
Photodynamic therapy (PDT) is an emerging clinical modality for treatment of neoplastic and non-malignant lesions. Applications of PDT require a photosensitizing drug, light, and oxygen. A series of photochemical reactions generate singlet oxygen from the 3O2 causing tissue damage in the regions where these three key components come together.1,2 This is a highly localized effect because the half-life of singlet oxygen is low (0.6 × 10−6 s).3 In cancer treatment, PDT can destroy the vasculature surrounding tumour cells, and activates immunological responses against them.4 The main attribute of PDT is its potential for dual selectivity, i.e. preferential accumulation of the photosensitizer in diseased – over normal – tissues, and focusing light to confine damage to the targeted region.5 PDT is relatively non-invasive, and treatments can be repeated without induction of resistance.2One of the earliest clinical PDT agents is porfimer sodium (Photofrin®), a purified hematoporphyrin derivative. Porfimer sodium is a mixture of oligomeric porphyrin units (up to eight) linked by esters and ethers. It has received worldwide regulatory approval in several indications, including cancers of the esophagus, lung and bladder. Porfimer sodium is activated by red light at ca. 630 nm. Photons of this wavelength do not penetrate tissue beyond a few millimeters, hence porfimer sodium is only suitable for superficial tumours, or ones that can be reached via endoscopic/fiber optic procedures. Moreover, porfimer sodium has a low absorbance at 630 nm necessitating extended irradiation from a high-energy source, and this often leads to complications. Another disadvantage of porfimer sodium is that it is not cleared quickly leading to post-treatment skin photosensitivity.6
Recognition of the disadvantages of porfimer sodium has inspired efforts to develop more effective PDT photosensitizers. Desirable properties7,8 for such agents include:
• low toxicity in the absence of light;
• low side-effect profiles (e.g. skin photosensitivity and pain after irradiation);
• appropriate lipophilic/hydrophilic balance for selective accumulation in tumour tissue;
• high extinction coefficients, particularly at long wavelengths for deep tissue penetration of light;
• low quantum yields for photobleaching; and
• high singlet-to-triplet intersystem crossing efficiencies.
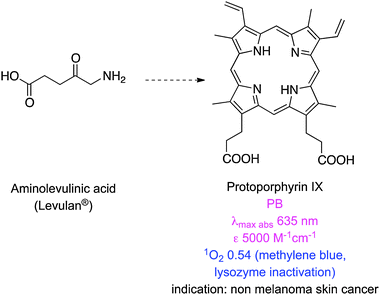
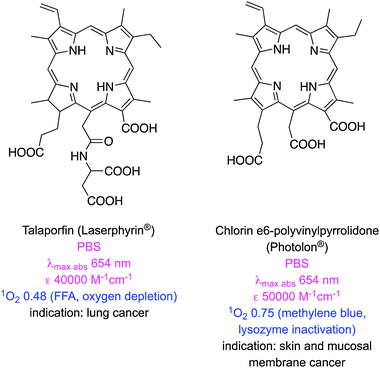
Table 1 lists some newer photosensitizers that have been approved for anti-cancer PDT along with some of their salient physicochemical properties (comprehensive reviews on these compounds have been published elsewhere).8 One of these clinically applied photosensitizers, 5-aminolevulinic acid (ALA), is not a chromophore, but a precursor for the biosynthesis of the protoporphyrin IX (PpIX).9 In tumours expression of ferrochelatase, the enzyme that converts PpIX to heme, is downregulated causing accumulation of the protoporphyrin PDT agent.10 PpIX is rapidly cleared from the body, minimizing the risk of skin photosensitization.11 However, ALA is hydrophilic and has limited penetration across certain biological barriers, so a lipophilic derivative, methyl-aminolevulinic acid, has also been developed.12 Nevertheless, the absorption spectrum of PpIX at 630 nm is similar to that of porfimer sodium hence it also gives only superficial tissue penetration in PDT.
| Photosensitizer | λ max abs (nm) | ε (M−1 cm−1) | λ max emiss (nm) | Φ Fl | Φ PB | Φ Δ | Log Po/w |
|---|---|---|---|---|---|---|---|
| Porfimer sodium (Photofrin®) | 63013 | 300013 | NA | NA | 5.4 × 10−5 (PB)14 | 0.25 (PB + 1% TX100; 630 nm; oxygen depletion with FFA)15 | 3.96 (calc.)16 |
| Protoporphyrin IX (Levulan®) | 63513 | 500013 | 630 (ex 397 nm; PBS)17 | 0.011 (ex 397 nm; PBS)17 | NA | 0.54 (PB + 1% TX100; 630 nm; lysozyme inactivation; RB at 0.75)18 | NA |
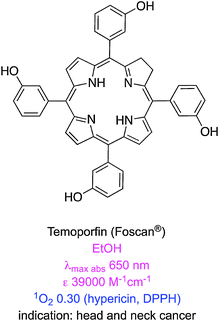
| Temoporfin (Foscan®) | 650 (EtOH)19 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (EtOH)19 000 (EtOH)19 |
655 (PBS)20 | NA | 1.54 × 10−5 (PBS + 10% | 0.31 (PBS + 10% FCS; >610 nm; DPBF; hypericin at 0.36)21 | 9.2422 |
| 652 (H2O)19 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (H2O)19 000 (H2O)19 |
FCS)21 | |||||
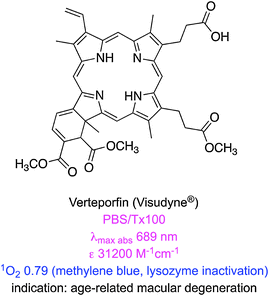
| Verteporfin (Visudyne®) | 688 (PBS + 2% TX100)23 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (PBS + 2% TX100)23 200 (PBS + 2% TX100)23 |
694 (PBS + 2% TX100)23 | 0.049 (PBS + 2% TX100)23 | 5.35 × 10−5 (PBS + 2% TX100)23 | 0.82 (PB + 1% TX100; 692 nm; lysozyme inactivation; MB at 0.52)18 | 7.76 (calc.)24 |
| 692 (PBS)23 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 (PBS)23 500 (PBS)23 |
695 (PBS)23 | 0.002 (PBS)23 | 2.80 × 10−5 (PBS)23 | |||
| Talaporfin (Laserphyrin®) | 654 (PBS)25 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (PBS)25 000 (PBS)25 |
660 (PBS)25 | NA | 8.2 × 10−4 (PBS)25 | 0.77 (D2O, oxygen depletion with FFA)25 | −1.9226 |
| Ce6 (Photolon®) | 663 (PBS)27 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (PBS)27 000 (PBS)27 |
662 (PBS)27 | 0.18 (PBS)27 | NA | 0.75 (PB; 660 nm; lysozyme inactivation; MB at 0.52)18 | 0.7827 |
There are some clinically approved chlorin-based photosensitizers that are similar to PpIX. One of these, temoporfin (Foscan®), offers improved potency, less skin photosensitivity, and a longer maximum absorption wavelength.28 However, temoporfin is so hydrophobic that it can precipitate upon administration.29 Similarly verteporfin is activated by light at 690 nm, clears rapidly from the body, and only generates short-term skin photosensitivity.30 This agent self-aggregates in aqueous solution,31 hence it is applied in liposome formulations; this mode of delivery restricts the scope of use to, so far, age related macular degeneration caused by abnormal blood vessel growth of the retina.32 Two other clinically approved chlorin-based photosensitizers are mono-aspartyl-L-chlorin e6 (also known as talaporfin) and chlorin e6-polyvinylpyrrolidone. Both these PDT agents are excellent singlet oxygen generators but have high photobleaching rates that reduce their PDT efficiencies.33
The discussion above correctly implies that most of the clinically relevant PDT agents are cyclic tetrapyrroles (porphyrins, chlorins, and bacteriochlorins).34,35 These can be synthetically inaccessible, and modifications to modulate their photophysical and biological properties are correspondingly difficult. Consequently, there is interest in non-porphyrin photosensitizers that might be made more easily.36–38 Phenothiazinium-based structures are a well-known category of this type of PDT agent; they are easy to make but have low light-to-dark toxicity ratios.39
A new class of PDT agents has emerged over the past decade: these are based on the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) core. BODIPYs have many ideal photosensitizer characteristics including high extinction coefficients, environment insensitivity, resistance to photobleaching,40 and higher light–dark toxicity ratios41 than phenothiazinium39 PDT agents. Several review papers have covered the role of BODIPYs as fluorescence imaging probes,42–46 but none have focused on derivatives for PDT. Fluorescence occurs via relaxation from singlet excited states, so high quantum yields for fluorescence are undesirable since this means that much of the energy absorbed on excitation does not cross to triplet states. Consequently, BODIPYs for PDT have to be modified to depress fluorescence and enhance singlet-to-triplet intersystem crossing. This review summarizes characteristics of selected members in this emerging class of BODIPY-based PDT agents.
Halogenated BODIPYs
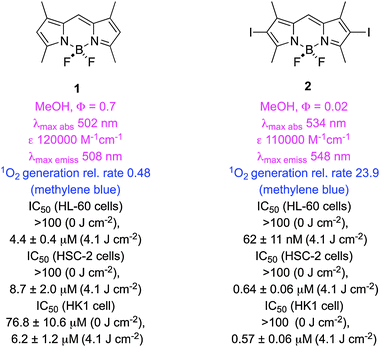
BODIPY derivatives are amenable to extensive modifications around the 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene core. Most of the dyes in this class have many ideal characteristics of PDT agents (low dark toxicities, cellular uptake, high extinction coefficients, low quantum yields for photobleaching) hence modifications are possible that enable absorbance at long wavelengths. However, most BODIPY dyes are efficiently excited into higher level singlet states, fluoresce from these, and do not cross to triplets; in fact, observation of triplet excited states in BODIPY dyes can be regarded as a novelty.47,48 Photo-damage in PDT is thought to occur predominantly via triplet excited states, consequently BODIPY dyes for PDT tend to be modified to enhance intersystem crossing (ISC). Spin-coupling to heavy atoms is the most common of these modifications (the “heavy atom effect”), and the one most frequently encountered is halogenation. Appropriate placing of heavy atoms on the BODIPY core promotes spin–orbit coupling, hence ISC, but not energy loss from excited states. Heavy atoms are not typically added at positions that could disrupt the planarity of the dye as this would decrease conjugation.“Tetramethyl-BODIPY” 1 does not contain a halogen, or significantly populate triplet states on excitation, and has a poor quantum yield (QY) for singlet oxygen (1O2) generation. Nagano's group was first to investigate a diiodo-analog, 2, for singlet oxygen generation in PDT.40 Formation of 1O2 was inferred via a near IR absorbance at 1268 nm that emerged when 2 was excited at 514 nm. Measurements of rate and QY for 1O2-generation in a standard way, using 1,3-diphenylisobenzofuran (DBPF), revealed high efficiencies for this process in both polar and apolar solvents. Unsurprisingly, then, compound 2 was shown to have high light-to-dark photocytotoxicity ratios (HeLa cells). Nagano et al. suggested that high oxidation potentials are desirable because they may protect BODIPY from self-oxidation. They also argued that there are potential applications of PDT in membranes; apolar dyes like 2 are useful for studying effects in lipophilic media like this.
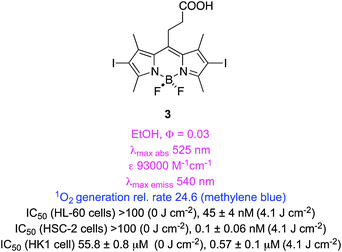
Two studies have compared singlet oxygen generation of a range of iodinated BODIPYs. In the first,41 iodination of meso-aryl substituents was found to have less impact than that of core-attached iodines. However, simple incorporation of a meso-ethylene-carboxylic acid group as in 3 improved the rate of singlet oxygen generation and light-induced photocytotoxicities (two of three cell lines, the other was the same over 2). BODIPY 3 was found to localize in the mitochondria of HSC-2 cells, and to induce G2/M arrest about 2 h after irradiation caused apoptosis. In general the physical parameters for singlet oxygen generation in this series correlated with their light-induced photocytotoxicities; this is noteworthy because such correlations are not always observed.
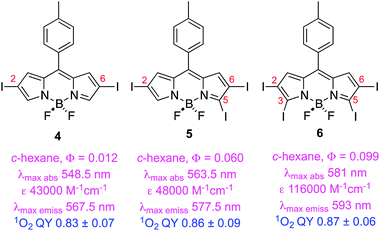
The second study of iodinated BODIPYs involved compounds like 4–649 having iodine atoms at different positions around the BODIPY core, and measurement of QYs of oxygen generation for selected compounds. Surprisingly, introduction of iodines at the 3- and 5-positions increases fluorescence. Flash photolysis experiments showed monoexponential decay of the excited states of these dyes, consistent with predominant recovery to the starting material state indicative of high stability against photobleaching.
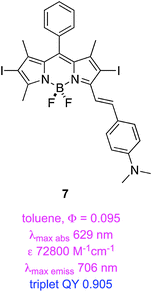
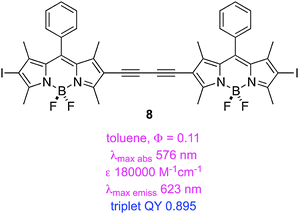
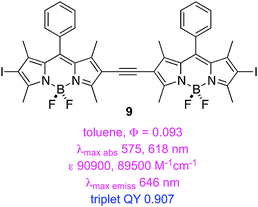
Triplet excited states for BODIPY dyes are pertinent to triplet–triplet annihilation, hence some groups have studied iodinated systems like the styryl-substituted one, 7, and the dimers 8–9.50 Triplet lifetimes indicated for these structures were measured via time-resolved spectroscopy. Estimates of triplet quantum QY were quoted based on 1 − (fluorescence QY), but this is an overestimate because it assumes that non-radiative decay processes are not operative.
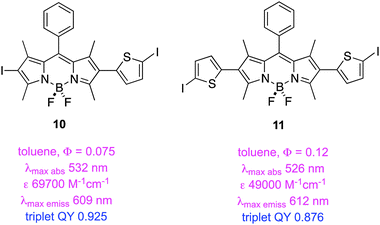
A second study from Zhao and Li featured insertion of thiophene units between the iodine and the BODIPY core. This gave dyes 10 and 11 that have exceptionally long triplet lifetimes, slight red-shifted absorption and fluorescence maxima, and markedly decreased extinction coefficients. These dyes also exhibit significant fluorescence indicating incomplete ISC.51 Data specifically relating to the PDT properties of 7–11 have not been reported.
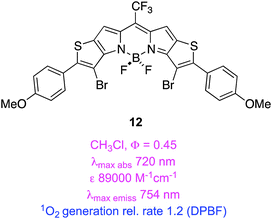
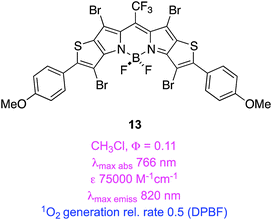
Thiophene is less aromatic than benzene, and its HOMO/LUMO energy levels are more suitable for conjugation with some unsaturated fragments. Extended heterocycles containing thiophene fragments can have similar useful characteristics. For instance, in 12 and 13 the heteroaryl-fused “KFL-4” BODIPY cores52,53 have long wavelength absorption maxima, high molar extinction coefficients, high QYs for 1O2 generation, and have higher photostabilities than the clinically approved PDT agent (mTHPC). Moreover, these brominated compounds have residual fluorescence that might enable them to be used simultaneously for imaging and PDT.
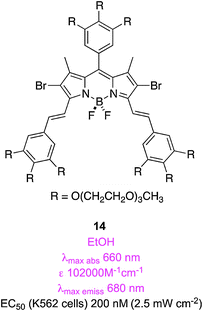
Compound 7 above is an example of a “styryl-substituted” BODIPY. Akkaya's group, pioneers of this area, showed compounds like this are conveniently formed via Knoevenagel reactions since 2,7-methyl substituents on BODIPYs are slightly acidic.54–57 In the first contribution on the PDT characteristics of these compounds, Akkaya's team made three brominated systems that also have oligoethylene glycol fragments to promote water-solubilities.58 Compound 14 was the most studied of these; it had an EC50 (conc. required for 50% of the maximal effect; excitation at 625 nm) of 200 nM and the cytotoxicity was attributed to cell-membrane damage as indicated via fluorescence microscopy.
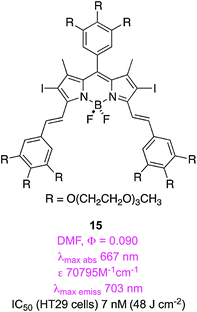
In a similar study, but featuring diiodo-BODIPY dyes, Ng and co-workers found that 15 was the most promising of four related potential PDT agents. They implied that this had the lowest EC50 in the series (7 nM on HT29 carcinoma cells) possibly because it permeated into cells, and accumulated inside, giving the most intense fluorescence. Fluorescence microscopy experiments indicated that this compound localized in the endoplasmic reticulum (ER, an organelle involved in lipid and protein synthesis).
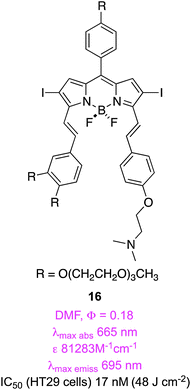
The research on compound 15 described above was followed by more studies on styryl-substituted BODIPYs, but this time on ones with two different substituents. It was hypothesized that the unsymmetrical substitution pattern would promote amphiphilic character.59 Dimethylamine 16 was the most studied in this series; it had a low EC50 (17 nM) and localized in lysosomes, less in mitochondria, and, unlike 15, not in the ER. Overall, the authors concluded that the functional groups on the alkene were more important to the localization behavior of the dyes than the lack of symmetry in the system. This paper is an excellent reference for data on standards for singlet oxygen generation.6,60
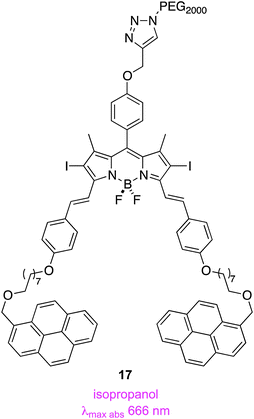
An attractive feature of Akkaya's route to styryl-substituted dyes is the diversity of aromatic aldehydes that can be condensed to obtain these products. For instance, the pyrene-containing systems 17 were prepared to facilitate non-covalent, supramolecular interactions between these compounds and single-walled carbon nanotubes. Nanotubes of this kind are internalized by mammalian cells, hence their interaction with the pyrene potentially could be used for intracellular delivery of the PDT agent. Complexation of the nanotubes with the agent was, in the event, observed and accompanied by a small decrease in the singlet oxygen generation efficiency, but cytotoxicity studies have not been reported so far.
Halogenated aza-BODIPY PDT agents
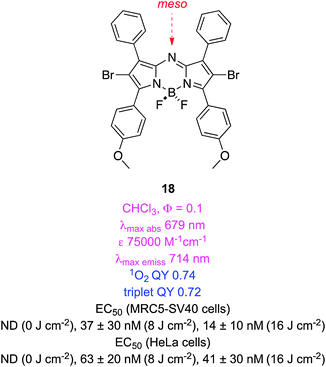
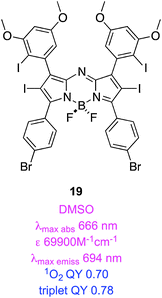
Aza-BODIPYs like 1861,62 have the BODIPY meso-carbon substituted by nitrogen, and this has some surprising effects. Notably, aza-BODIPYs have absorbance and fluorescence emissions of around 650 and 675 nm, and these may be displaced to even longer wavelengths in compounds containing an electron donating group para-oriented relative to the alkene (e.g. OMe in 18). Bromination of aza-BODIPY 2,6-positions results in at least a four-fold reduction in fluorescence QY, and an increased population of triplet states upon excitation giving at least 1000× differences between light and dark cytotoxicities. That para-substituent also modulates PDT activity; for instance, the corresponding system without the methoxide generated less singlet oxygen than 18, even when present at 100× the concentration. Molar extinction coefficients of these systems are significantly better than those of porphyrins. Unfortunately, the aqueous solubilities of aza-BODIPYs tend to be modest so they are often delivered in cellular assays using a cremophor (a common excipient used in drugs to increase water solubilities, cf. a cremophor is used in formulations of paclitaxel for the same reason).Compound 18 has a QY for singlet oxygen generation of 74%.63 Time resolved spectroscopy revealed that its triplet quantum yield was 72% (a lifetime of 21 μs) and that the dye was exceptionally photostable.63 The tetraiododibromo derivative 19 had a similar triplet QY (78%; a lifetime of 1.6 μs).64 Quantum mechanical calculations (DFT) on these systems have been used to understand their HOMO–LUMO levels and singlet-to-triplet energy gaps.65
Dibromo-aza-BODIPY 18 (designated ADPM06 in papers) has been extensively studied in cells and in vivo assays. It has a nanomolar EC50 for light-induced cytotoxicity in a range of different human tumour cell lines, with no discernable selectivity for any particular type. Encouragingly, these cell types include some drug-resistant and metastatic lines. Cells can die via necrotic or apoptotic pathways; 18 administered at EC50 concentrations caused apoptotic cell death. Moreover, even though cell death in PDT can be reduced under depleted oxygen levels (e.g. hypoxia in cancer cells), 18 retained significant activity under these conditions.60 Apoptosis is initiated in PDT mediated by 18 as a result of active oxygen species generated around the ER. This is accompanied by activation of several inhibitor and executioner caspases. Positron emission tomography studies using 18F-labeled agents showed that a marked decrease in tumor proliferation (breast and glioma models) occurred 24 h post-PDT treatment with 18.60 In fact, ablation of breast tumors was observed in 71% of mice treated with 18 at 2 mg kg−1 after irradiation; this is comparable to “cure-rates” for more established PDT agents in mice xenograft models. The inherent fluorescence of 18 facilitated studies to determine the organ distribution and clearance of this compound; the data are consistent with that of an ideal initiator of PDT. There was no accumulation of 18 in the skin, an important property for PDT agents. Positron emission tomography and magnetic imaging studies showed that this PDT agent caused a decrease in tumour-vasculature perfusion and -metabolic activity.66
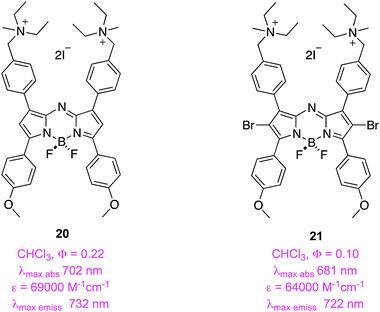
Applications of PDT are not limited to chemotherapy of cancer; another, though rarer, use of these agents is as anti-bacterials. O'Shea and co-workers hypothesized that the quaternary ammonium salt 20 may implant into bacterial membranes as a result of its positive charge and amphiphilic character. Fluorescence studies with the non-halogenated analog 20 demonstrated that this stains both Gram-negative (E. coli) and -positive (S. aureus) bacteria, and yeast cells (C. albicans) with a bias to the membrane regions. Encouragingly, a human cell line (MDA-MB-231) showed only minimal uptake in the same timeframe. Strong antibacterial activity on these microbes was observed when they were irradiated with 21; total eradication occurred at concentrations of 1–5 μg mL−1.
PDT characteristics modulated by photoinduced electron transfer (PET)
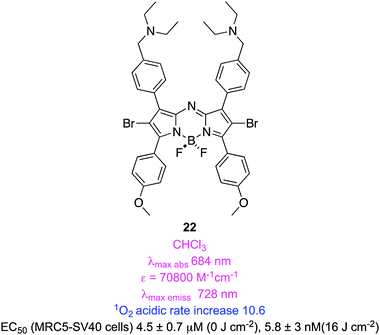
Several groups converged on the idea that photoinduced electron transfer (PET; unfortunately, this is also a widely used abbreviation for positron emission tomography) can be used to selectively quench intersystem crossing to triplet states. They have applied this hypothesis in several different and ingenious ways.Some PDT side-effects may arise from prolonged light sensitivity. O'Shea recognized that aza-BODIPY dyes with a non-conjugated but proximal amine may undergo rapid relaxation via PET processes when the amine is not protonated. However, a larger portion of the amine would be protonated in the relatively acidic (pH 6.5–6.8) interstitial fluid that surrounds tumours, PET would selectively diminish in those regions, and the cytotoxic effect would be greater around cancerous cells than healthy ones.67 Dye 22 was the pivotal one used to investigate this hypothesis. This agent was shown to generate more singlet oxygen in acidic than in neutral media, and an EC50 value of 5.8 nM was recorded for light-induced cytoxicity. However, to the best of our knowledge, photocytotoxicities of this agent in vivo have not yet been compared with closely related compounds that lack the amine groups, so the clinical potential of 22 is still an open question.
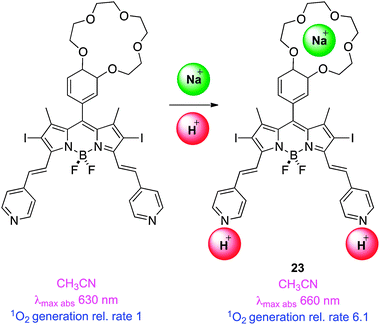
Another way to use PET modulation of singlet-to-triplet conversion is via an appropriately situated crown ether.68 Intracellular sodium ion concentrations are apparently around 3× higher in tumor cells than in healthy ones, so coordination of these to a crown might selectively increase the PET effect in tumour cells. Thus Akkaya and co-workers combined meso-crown ether with pyridyl–styryl substituents in molecule 23 to sense higher sodium ion and proton concentrations in tumour cells, respectively. The authors observed cumulative effects of both stimuli in singlet oxygen generation, but conceded that the concentrations required to achieve a desirable response were greater than intracellular levels; no cell studies were reported.69
An insightful assertion by Nagano et al. was mentioned earlier in this review: that electron withdrawing BODIPY-substituents should protect BODIPYs from oxidation. A recent study from that group featured a range of BODIPY dyes with different electron withdrawing groups at the 2- and 6-positions.70 Observation of singlet oxygen production confirmed that these dyes are most stable with electron withdrawing groups. A rough inverse correlation between levels of singlet oxygen production and the electron withdrawing abilities of these substituents was also noted. Observed QYs for singlet oxygen generation were probably not high on an absolute scale (the paper did not mention what they were) but the study does point to a fundamental issue: singlet oxygen generation can be modulated by tuning the oxidation potential of the BODIPY core. This concept was used very effectively in the next study from the Nagano lab, described below.
All the applications of PDT so far target cells as a whole, wherein the mechanisms by which the cell biology is disrupted are not of primary importance.71 On a molecular scale, however, it is possible to use highly localized singlet oxygen generation to disable specific proteins; this is the technique of chromophore assisted light inactivation (CALI). Nagano's group had the idea that a hydrophobic BODIPY-based sensitizer might bury itself in a lipophilic cavity of a protein receptor when brought into proximity via binding to a conjugated ligand. This strategy is likely to be most effective when singlet oxygen production is enhanced by placing the sensitizer in a lipophilic environment. The specific case studied was inositol 1,4,5-trisphosphate (IP3) coupled to a 2,6-diiodo-BODIPY; the hypothesis was that ligand binding would place the dye into a hydrophobic cavity that is easily seen in the receptor that binds IP3 (IP3R). They showed that the electron donating substituent in structure 25 modulated the properties of the sensitizer such that the production of singlet oxygen was slow except in relatively apolar solvents.
An attribute of this particular system is that binding to IP3R gives a measurable biochemical output, i.e. increased Ca2+ concentration. Thus binding of the 2,6-diiodo-BODIPY conjugate gave dose-dependent release of Ca2+ with an EC50 value of 3 μM, while 2,6-diiodo-BODIPY conjugated with the enantiomeric IP3-ligand did not give the same Ca2+ release. Permeablized cells were then used to input a calcium ion sensor and the appropriate conjugates; only the ones with an environment-activated photosensitizer conjugated to the appropriate IP3-ligand enantiomer gave calcium release that was negatively modulated by treatment with light (Fig. 1).
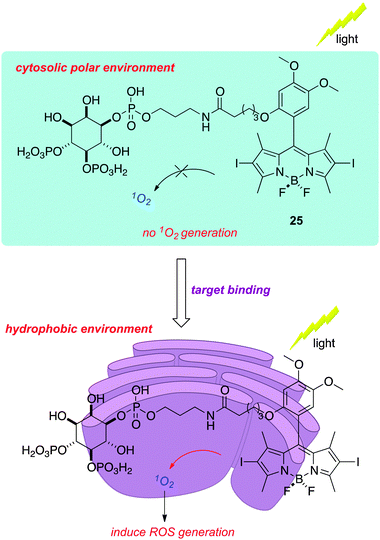 | ||
| Fig. 1 Binding of a functionalized BODIPY to the inositol 1,4,5-trisphosphate receptor places the PDT agent in a hydrophobic environment where singlet oxygen generation is favored, leading to inactivation of the protein. | ||
Halogen-free BODIPY sensitizers
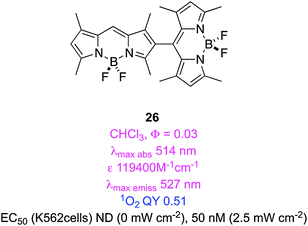
There is nothing special about halogen atoms in design of BODIPY derivatives for triplet-sensitization; any substituent with molecular orbitals having appropriate multiplicity and energy levels might function in this way. Surprisingly, some BODIPY fragments have emerged as appropriate substituents to induce triplet-sensitization. Thus, dimers of BODIPY dyes wherein the chromophores are directly connected may, on excitation, undergo more efficient ISC to triplet states than the corresponding monomers.72Computational studies have been used to predict orthogonal chromophores that may give electronic mixing in the excited states to generate triplets. Selection of the appropriate computational method is important; here multiconfigurational self-consistent field, MCSFF, was used. Just as predicted, bisBODIPY systems like 26 were less fluorescent than their constituent monomers, and gave relatively high singlet QYs. An EC50 of 50 nM was measured for human erythroleukemia cells (Fig. 2).73
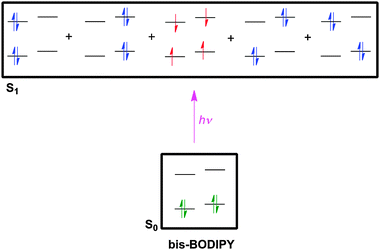 | ||
| Fig. 2 Excitation of bisBODIPY systems like 26 gives singlet excited states (blue electrons), but a triplet state (red) is also favored. | ||
Attempts to extend conjugation using the styryl approach failed to give triplet oxygen production at higher wavelengths. We suggest that this could be due to accelerated photobleaching of a long-lived triplet excited state.74
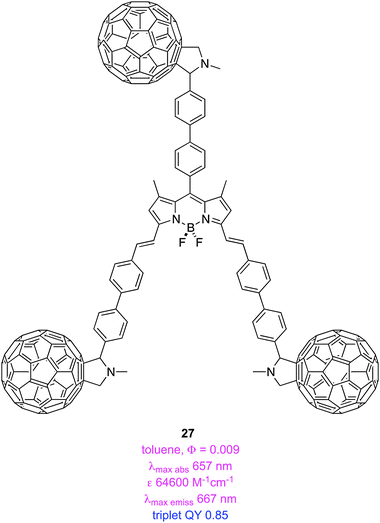
BODIPY derivative 27 is an organic triplet photosensitizer; it is particularly interesting because no halogens or other heavier elements are involved.75 It appears that the BODIPY fluorescence is quenched via intramolecular energy transfer to the styryl protected C60-dyads, accounting for the long-lived triplet excited state (123.2 μs) of this material.
BODIPY dyes for observing reactive oxygen species
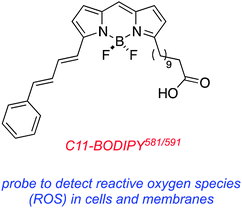
There are BODIPY-derivatives designed to be sensors for the generation of reactive oxygen species. These are not necessarily PDT agents, but they can be used to monitor consequences of PDT treatment. One useful probe of this kind is the commercially available C11-BODIPY. For example, this probe was used to demonstrate that oxidants were present in a cell culture up to 30 min after illumination in a PDT experiment. A dark control showed that hydrogen peroxide only activated the probe when it was in direct contact with the cells so the researchers were able to deduce that the reactive oxygen species involved in the PDT experiment were not confined to peroxide anions.76Conclusions
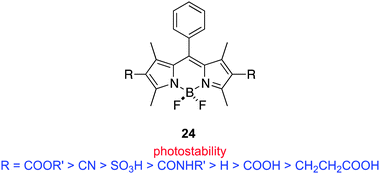
Many studies have focused on BODIPY core modifications to facilitate singlet oxygen generation. The intrinsic absorption maxima of simple BODIPY dyes (ca. 510–530 nm) are shorter than ideal, so many of the featured modifications also aim to extend conjugation in these molecules. For instance, Akkaya's methyl-BODIPY condensation reaction has been used several times, including studies by other groups, for this purpose. One of the most promising avenues of research, pioneered mainly by O'Shea, centers on aza-BODIPY compounds as PDT agents; these are less synthetically accessible, but have red-shifted absorption maxima. In our view, aza-BODIPY agents are probably closer to clinical development than any subcategory in the BODIPY class.An interesting consequence of the PDT work is Nagano's CALI technique to eliminate selected receptors on a molecular level. This approach is mostly intended for in vitro and cellular studies, so wavelength of absorption is not critical. BODIPY dyes can also be used as sensors for reactive oxygen species in studies involving other types of agents.
A priority for future research must be to develop clinically useful PDT agents. Possibly this will be coupled with active-targeting. Thus it will be interesting to see future studies featuring BODIPYs conjugated with ligands for cell-surface receptors that are over-expressed on tumour cells. It is surprising that we did not encounter reports of this strategy, even employing common small molecule targeting agents like RGD peptidomimetics and folic acid.
Abbreviations
| λ max abs | Absorption maxima (Q-band) |
| ε | Molar extinction coefficient |
| λ max emiss | Fluorescence emission maxima |
| Φ Fl | Fluorescence quantum yield |
| Φ PB | Photobleaching quantum yield |
| Φ Δ | Singlet oxygen generation quantum yield |
| Log Po/w | Log octanol/water partition coefficient |
| PB | Phosphate buffer pH ∼ 7.4 |
| EtOH | Ethanol |
| PBS | Phosphate buffered saline |
| TX100 | Triton X100 |
| FFA | Furfuryl alcohol |
| MB | Methylene blue |
| RB | Rose Bengal |
| NA | Not available |
Acknowledgements
We thank The National Institutes of Health (GM087981), The Robert A. Welch Foundation (A-1121), and HIR-MOHE grant (UM.C/625/1/HIR/MOHE/MED/17), Ministry of Higher Education, Malaysia, for financial support.References
- P. Agostinis, K. Berg, A. Cengel Keith, H. Foster Thomas, W. Girotti Albert, O. Gollnick Sandra, M. Hahn Stephen, R. Hamblin Michael, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, C. Wilson Brian and J. Golab, CA Cancer J. Clin., 2011, 61, 250–281 CrossRef
.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS
.
- J. Moan and K. Berg, Photochem. Photobiol., 1991, 53, 549–553 CrossRef CAS
.
- A. Juarranz, P. Jaen, F. Sanz-Rodriguez, J. Cuevas and S. Gonzalez, Clin. Transl. Oncol., 2008, 10, 148–154 CrossRef CAS
.
- A. P. Castano, T. N. Demidova and M. R. Hamblin, Photodiagn. Photodyn. Ther., 2004, 1, 279–293 CrossRef CAS
.
- A. Hajri, S. Wack, C. Meyer, M. K. Smith, C. Leberquier, M. Kedinger and M. Aprahamian, Photochem. Photobiol., 2002, 75, 140–148 CrossRef CAS
.
- M. J. Garland, C. M. Cassidy, D. Woolfson and R. F. Donnelly, Future Med. Chem., 2009, 1, 667–691 CrossRef CAS
.
- R. R. Allison and C. H. Sibata, Photodiagn. Photodyn. Ther., 2010, 7, 61–75 CrossRef CAS
.
- J. C. Kennedy and R. H. Pottier, J. Photochem. Photobiol., B, 1992, 14, 275–292 CrossRef CAS
.
- M. Wachowska, A. Muchowicz, M. Firczuk, M. Gabrysiak, M. Winiarska, M. Wańczyk, K. Bojarczuk and J. Golab, Molecules, 2011, 16, 4140–4164 CrossRef CAS
.
- J. Webber, D. Kessel and D. Fromm, J. Photochem. Photobiol., B, 1997, 37, 151–153 CrossRef CAS
.
- A. Godal, N. O. Nilsen, J. Klaveness, J. E. Branden, J. M. Nesland and Q. Peng, J. Environ. Pathol., Toxicol. Oncol., 2006, 25, 109–126 CrossRef CAS
.
- W. M. Sharman, C. M. Allen and J. E. van Lier, Drug Discovery Today, 1999, 4, 507–517 CrossRef CAS
.
- J. D. Spikes, Photochem. Photobiol., 1992, 55, 797–808 CrossRef CAS
.
- S. Kimel, B. J. Tromberg, W. G. Roberts and M. W. Berns, Photochem. Photobiol., 1989, 50, 175–183 CrossRef CAS
.
- M. Tanaka, M. Kinoshita, Y. Yoshihara, N. Shinomiya, S. Seki, K. Nemoto, T. Hirayama, T. Dai, L. Huang, M. R. Hamblin and Y. Morimoto, Photochem. Photobiol., 2012, 88, 227–232 CrossRef CAS
.
- G. I. Lozovaya, Z. Masinovsky and A. A. Sivash, Origins Life Evol. Biospheres, 1990, 20, 321–330 CrossRef CAS
.
- J. M. Fernandez, M. D. Bilgin and L. I. Grossweiner, J. Photochem. Photobiol., B, 1997, 37, 131–140 CrossRef CAS
.
- M. F. Grahn, A. McGuinness, R. Benzie, R. Boyle, M. L. de Jode, M. G. Dilkes, B. Abbas and N. S. Williams, J. Photochem. Photobiol., B, 1997, 37, 261–266 CrossRef CAS
.
- I. Belitchenko, V. Melnikova, L. Bezdetnaya, H. Rezzoug, J. L. Merlin, A. Potapenko and F. Guillemin, Photochem. Photobiol., 1998, 67, 584–590 CrossRef CAS
.
- C. Hadjur, N. Lange, J. Rebstein, P. Monnier, H. van den Bergh and G. Wagnieres, J. Photochem. Photobiol., B, 1998, 45, 170–178 CrossRef CAS
.
- M. Chen, X. Liu and A. Fahr, Int. J. Pharm., 2011, 408, 223–234 CrossRef CAS
.
- B. Aveline, T. Hasan and R. W. Redmond, Photochem. Photobiol., 1994, 59, 328–335 CrossRef CAS
.
- J. K. Macalpine, R. Boch and D. Dolphin, J. Porphyrins Phthalocyanines, 2002, 6, 146–155 CrossRef CAS
.
- J. D. Spikes and J. C. Bommer, J. Photochem. Photobiol., B, 1993, 17, 135–143 CrossRef CAS
.
- D. Kessel, Photochem. Photobiol., 1989, 49, 447–452 CrossRef CAS
.
- H. A. Isakau, M. V. Parkhats, V. N. Knyukshto, B. M. Dzhagarov, E. P. Petrov and P. T. Petrov, J. Photochem. Photobiol., B, 2008, 92, 165–174 CrossRef CAS
.
- M. O. Senge and J. C. Brandt, Photochem. Photobiol., 2011, 87, 1240–1296 CrossRef CAS
.
- T. Kiesslich, J. Berlanda, K. Plaetzer, B. Krammer and F. Berr, Photochem. Photobiol. Sci., 2007, 6, 619–627 CAS
.
- J. M. Houle and A. Strong, J. Clin. Pharmacol., 2002, 42, 547–557 CrossRef CAS
.
- B. M. Aveline, T. Hasan and R. W. Redmond, J. Photochem. Photobiol., B, 1995, 30, 161–169 CrossRef CAS
.
- A. F. Cruess, G. Zlateva, A. M. Pleil and B. Wirostko, Acta Ophthalmol., 2009, 87, 118–132 CrossRef CAS
.
- Y. Hongying, W. Fuyuan and Z. Zhiyi, Dyes Pigm., 1999, 43, 109–117 CrossRef
.
- E. D. Sternberg, D. Dolphin and C. Brückner, Tetrahedron, 1998, 54, 4151–4202 CrossRef CAS
.
- E. S. Nyman and P. H. Hynninen, J. Photochem. Photobiol., B, 2004, 73, 1–28 CrossRef CAS
.
- M. Wainwright and R. M. Giddens, Dyes Pigm., 2003, 57, 245–257 CrossRef CAS
.
- F. Harris, L. K. Chatfield and D. A. Phoenix, Curr. Drug Targets, 2005, 6, 615–627 CrossRef CAS
.
- M. Wainwright, Photodiagn. Photodyn. Ther., 2005, 2, 263–272 CrossRef CAS
.
- M. Wainwright, D. A. Phoenix, L. Rice, S. M. Burrow and J. Waring, J. Photochem. Photobiol., B, 1997, 40, 233–239 CrossRef CAS
.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS
.
- S. H. Lim, C. Thivierge, P. Nowak-Sliwinska, J. Han, H. Van den Bergh, G. Wagnieres, K. Burgess and H. B. Lee, J. Med. Chem., 2010, 53, 2865–2874 CrossRef CAS
.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS
.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC
.
-
A. Loudet and K. Burgess, in Handbook of Porphyrin Science With Applications to Chemistry, Physics, Materials Science, Engineering, Biology and Medicine, ed. K. Kadish, K. Smith and R. Guilard, World Scientific, 2010, p. 203 Search PubMed
.
- G. Ulrich, R. Ziessel and A. Harriman, Angew.
Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS
.
- R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC
.
- B. Hinkeldey, A. Schmitt and G. Jung, ChemPhysChem, 2008, 9, 2019–2027 CrossRef CAS
.
- T. N. Singh-Rachford, A. Haefele, R. Ziessel and F. N. Castellano, J. Am. Chem. Soc., 2008, 130, 16164–16165 CrossRef CAS
.
- M. J. Ortiz, A. R. Agarrabeitia, G. Duran-Sampedro, J. Banuelos Prieto, T. A. Lopez, W. A. Massad, H. A. Montejano, N. A. Garcia and I. Lopez Arbeloa, Tetrahedron, 2012, 68, 1153–1162 CrossRef CAS
.
- W. Wu, H. Guo, W. Wu, S. Ji and J. Zhao, J. Org. Chem., 2011, 76, 7056–7064 CrossRef CAS
.
- Y. Chen, J. Zhao, L. Xie, H. Guo and Q. Li, RSC Adv., 2012, 2, 3942–3953 RSC
.
- K. Umezawa, A. Matsui, Y. Nakamura, D. Citterio and K. Suzuki, Chem.–Eur. J., 2009, 15, 1096–1106 CrossRef CAS
.
- K. Umezawa, Y. Nakamura, H. Makino, D. Citterio and K. Suzuki, J. Am. Chem. Soc., 2008, 130, 1550–1551 CrossRef CAS
.
- A. Coskun, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2007, 9, 607–609 CrossRef CAS
.
- E. Deniz, G. C. Isbasar, O. A. Bozdemir, L. T. Yildirim, A. Siemiarczuk and E. U. Akkaya, Org. Lett., 2008, 10, 3401–3403 CrossRef CAS
.
- Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461–464 CrossRef CAS
.
- O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644–4647 CrossRef CAS
.
- S. Atilgan, Z. Ekmekci, A. L. Dogan, D. Guc and U. Akkaya Engin, Chem. Commun., 2006, 4398–4400 RSC
.
- H. He, P.-C. Lo, S.-L. Yeung, W.-P. Fong and D. K. P. Ng, Chem. Commun., 2011, 47, 4748–4750 RSC
.
- W. M. Gallagher, L. T. Allen, C. O'Shea, T. Kenna, M. Hall, A. Gorman, J. Killoran and D. F. O'Shea, Br. J. Cancer, 2005, 92, 1702–1710 CrossRef CAS
.
- J. Killoran, L. Allen, J. F. Gallagher, W. M. Gallagher and D. F. O'Shea, Chem. Commun., 2002, 1862–1863 RSC
.
- A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619–10631 CrossRef CAS
.
- P. Batat, M. Cantuel, G. Jonusauskas, L. Scarpantonio, A. Palma, D. F. O'Shea and N. D. McClenaghan, J. Phys. Chem. A, 2011, 115, 14034–14039 CrossRef CAS
.
- N. Adarsh, R. R. Avirah and D. Ramaiah, Org. Lett., 2010, 12, 5720–5723 CrossRef CAS
.
- A. D. Quartarolo, N. Russo and E. Sicilia, Chemistry, 2006, 12, 6797–6803 CrossRef CAS
.
- A. T. Byrne, A. E. O'Connor, M. Hall, J. Murtagh, K. O'Neill, K. M. Curran, K. Mongrain, J. A. Rousseau, R. Lecomte, S. McGee, J. J. Callanan, D. F. O'Shea and W. M. Gallagher, Br. J. Cancer, 2009, 101, 1565–1573 CrossRef CAS
.
- S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2005, 127, 16360–16361 CrossRef CAS
.
- O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029–8036 CrossRef CAS
.
- S. Ozlem and E. U. Akkaya, J. Am. Chem. Soc., 2008, 131, 48–49 CrossRef
.
- T. Komatsu, D. Oushiki, A. Takeda, M. Miyamura, T. Ueno, T. Terai, K. Hanaoka, Y. Urano, T. Mineno and T. Nagano, Chem. Commun., 2011, 47, 10055–10057 RSC
.
- T. Yogo, Y. Urano, A. Mizushima, H. Sunahara, T. Inoue, K. Hirose, M. Iino, K. Kikuchi and T. Nagano, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 28–32 CrossRef CAS
.
- B. Ventura, G. Marconi, M. Broering, R. Kruger and L. Flamigni, New J. Chem., 2009, 33, 428–438 RSC
.
- Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937–11941 CrossRef CAS
.
- S. Duman, Y. Cakmak, S. Kolemen, E. U. Akkaya and Y. Dede, J. Org. Chem., 2012, 77, 4516–4527 CrossRef CAS
.
- L. Huang, X. Yu, W. Wu and J. Zhao, Org. Lett., 2012, 14, 2594–2597 CrossRef CAS
.
- D. V. Sakharov, E. D. R. Elstak, B. Chernyak and K. W. A. Wirtz, FEBS Lett., 2005, 579, 1255–1260 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2013 |