A computational study of the origin of stereoinduction in NHC-catalyzed annulation reactions of α,β-unsaturated acyl azoliums†
Abstract
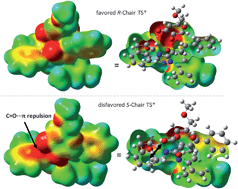
The origin of stereoselectivity of NHC-catalyzed annulation reactions of
- This article is part of the themed collection: Physical Chemistry

 Please wait while we load your content...
Please wait while we load your content...