Magnetically separable Fe3O4–Ag3PO4 sub-micrometre composite: facile synthesis, high visible light-driven photocatalytic efficiency, and good recyclability†
Gaiping
Li
and
Lanqun
Mao
*
Beijing National Laboratory for Molecular Sciences, Key Laboratory of Analytical Chemistry for Living Biosystems, Institute of Chemistry, the Chinese Academy of Sciences (CAS), Beijing, 100190, China. E-mail: lqmao@iccas.ac.cn.; Fax: +86-10-62559373
First published on 26th March 2012
Abstract
A magnetically separable Fe3O4–Ag3PO4 sub-micrometre composite was synthesized in large quantities by a fast and simple route, and was demonstrated to have a high photocatalytic efficiency toward the decomposition of methylene blue dye under visible light irradiation with a good recyclability.
Introduction
Photocatalysis has been attracting a growing interest because it provides a new promising way to meet the challenges of the environment, energy and sustainability.1,2 In the development of efficient photocatalytic systems, the synthesis of high-performance photocatalysts with good recyclabilities remains most important. Among a wide spectrum of the photocatalysts, titanium dioxide (TiO2) has attracted much attention over the past decades because of its excellent properties such as high photocatalytic efficiency, good stability, low cost, nontoxicity and so on.2,3 However, the wide band gap of TiO2 makes it only responsive to high-energy UV light, resulting in a low-efficiency in the utilization of solar energy. Such a limitation activated a great interest in developing new visible light-driven photocatalysts with high catalytic performances, which remain promising for practical applications.4–7Very recently, silver orthophosphate (Ag3PO4) has been put forward as a novel photocatalyst that exhibits extremely high photooxidative capabilities for the oxidation of water and the photodecomposition of organic dyes under visible-light irradiation.8–10 This excellent photocatalytic performance has been attributed partly to the highly dispersive Ag s-Ag s bands without localized d states, realizing a small effective mass of the electron, thus it can rapidly transfer to the surface prohibiting the carrier recombination.11 Current research along this line mainly lies in decreasing the size of Ag3PO4 down to the nanometre level12 since it is well known that the catalytic reaction mostly occurs on the surface of the catalysts, and thus the higher surface area of nanoparticles would be beneficial to the enhanced photocatalytic activity.13,14 Unfortunately, when the photocatalytic reaction is completed, it is often difficult to isolate and recover the nano-sized Ag3PO4 catalysts from the mixed system in a simple way, resulting in the second contamination.
Herein, we report a facile and fast approach for the synthesis of a magnetically separable composite based on Ag3PO4 and Fe3O4 in large quantities. The Ag3PO4–Fe3O4 sub-micrometre composites (denoted as AF MCs) are simply synthesized by the precipitation of Ag3PO4 on Fe3O4 nanoparticles under sonication in the absence of any capping agents. The resulting AF MCs have an excellent photocatalytic activity toward the decomposition of methylene blue (MB) dye in solution under visible light and natural sunlight irradiation. More importantly, they are easily isolated from the solution by an external magnet and are subsequently reused in competitive photocatalysis with a good recyclability.
Results and discussion
Porous magnetite (Fe3O4) nanospheres were synthesized by a solvothermal method, as reported previously15 (ESI†). The mean diameter of these Fe3O4 nanospheres was about 160 nm (Fig. S1 A†). Each magnetic microsphere was constructed with many small magnetic grains and the surface was rough (Fig. S1 B†). X-Ray diffraction (XRD) patterns of these Fe3O4 nanospheres confirm their face-centered cubic (fcc) structure (Fig. S1 C†). Additionally, the Fe3O4 nanospheres were well dispersed in water to form a black-brown dispersion and could be easily drawn to the sidewall by an external magnet (Fig. S1 D†), suggesting a good magnet-controlled property.Ag3PO4 was prepared by a simple precipitation process (see ESI† for experimental details), and a yellow milky solution was obtained (Fig. S2 A†). The particle size of the as-synthesized Ag3PO4 was about 100–450 nm with a smooth surface. The AF MCs were prepared in a similar precipitation process in the presence of Fe3O4 nanospheres. In order to determine the effect of the content of Ag3PO4 in the as-prepared AF MCs on the photocatalytic activity, three sets of samples with different quantities of Ag3PO4 were prepared by altering the amounts of Na2HPO4 and AgNO3 added into the aqueous dispersion of Fe3O4, where the molar ratio of AgNO3 to Na2HPO4 was kept to 3 (the stoichiometric ratio of Ag3PO4). It was found that, the resulting mixture became more and more turbid and some precipitation appeared when increasing the volume of the aqueous solutions of Na2HPO4 and AgNO3 added to the aqueous dispersion of Fe3O4. As expected, the as-prepared AF MCs exhibited a magnetic response (Fig. 1 A), confirming the successful attachment of Ag3PO4 to the Fe3O4 nanospheres. From the SEM (scanning electron microscope) images (Fig. 1 C), we found that, upon formation of the sub-micrometre composites, the Fe3O4 nanospheres and Ag3PO4 particles entangle each other to form structurally uniform hybrids (Fig. 1 B).
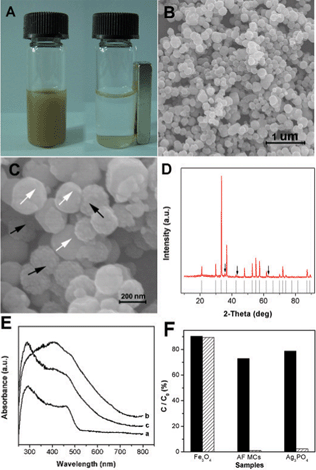 | ||
| Fig. 1 Photographs (A) of the AF MCs in sunlight, which were easily dispersed in water (left) and also could be drawn from the solution to the sidewall of the vial by an external magnet (right). SEM images (B and C) of the AF MCs at different magnifications. The white and black arrows in (C) indicate the Ag3PO4 and Fe3O4 nanospheres, respectively. XRD patterns (D) of the AF MCs and the standard Ag3PO4. The arrows indicate the Fe3O4 peaks. Ultraviolet-visible diffusive reflectance spectra (E) of the Ag3PO4 sub-micrometre particles (a), Fe3O4 nanospheres (b) and AF MCs (c). (F) The results on the adsorption ability (solid column) and photocatalytic activity (sparse column) of different samples under identical conditions. | ||
The photocatalytic activities of these three sets of samples with different Ag3PO4 content were evaluated by degrading the MB dye under visible light illumination without any sacrificial reagents. Prior to irradiation, the mixtures containing the MB dye and the samples were kept in the dark for 30 min to achieve an equilibrium adsorption of the MB dye on the surface of the particles. From the results depicted in Fig S2 F†, we could see that sample 2 adsorbs more MB dye (over 30%) and exhibits the best photocatalytic efficiency. Therefore, we chose sample 2 for the subsequent photocatalytic investigations.
The magnetic properties of pure Fe3O4 nanospheres and the AF MCs were studied using a vibrating sample magnetometer, and the hysteresis loops of the samples are listed in Fig. S3.† It can be seen that both samples exhibit negligible coercivity and remanence. The saturated magnetization (SM) values were 79.5 and 11.1 emu g−1 for the Fe3O4 nanospheres and the AF MCs, respectively. The decrease in the SM for the AF MCs could be explained by taking into account the diamagnetic contribution of the Ag3PO4 in the composites16,17 (the weight percentage of the Fe3O4 nanospheres in the composites was about 15 wt%).
Fig. 1B shows the SEM image of the AF MCs with the optimized content of Ag3PO4 for photocatalytic activity. It was possible to distinguish these two kinds of particles in the hybrid materials by their different surfaces as marked in Fig. 1 C. Fig. 1 D depicts the XRD pattern of the as-synthesized AF MCs. Most of the diffraction peaks match well with the body-centered cubic phase of Ag3PO4, and three smaller diffraction peaks were indexed to the fcc phase of Fe3O4, again confirming that both Fe3O4 and Ag3PO4 were present in the hybrid materials. Fig. 1 E displays the ultraviolet-visible diffuse reflectance spectra of pure Fe3O4 nanospheres, Ag3PO4 sub-micrometre particles and AF MCs. Consistent with the previous report,8 Ag3PO4 shows a strong absorption in the visible region with a wavelength shorter than 530 nm because of its smaller band gap. There was an obvious red-shift in the absorption edge of the AF MCs, which exhibit an enhanced absorption in the visible region compared to the Ag3PO4 sample. This property might have a positive contribution to the photocatalytic reactions18 because a more efficient utilization of the solar energy could be achieved.
In addition, the photocatalytic activities of pure Fe3O4 nanospheres, Ag3PO4 sub-micrometre particles, and AF MCs were also evaluated by decomposing MB dye under visible light illumination. As shown in Fig. 1 F, the pure Fe3O4 nanospheres could adsorb about 10% of the MB dye, whereas no obvious photocatalytic activity was detected. However, the AF MCs exhibit a high photocatalytic performance, which is slightly higher than that of the pure Ag3PO4 sample, suggesting that the incorporation of magnetic Fe3O4 into AF MCs might improve the photocatalytic activity presumably by enhancing the adsorption ability of MB and/or extending the absorption into the visible region.18–20 Therefore, the hybrid materials are beneficial for photocatalytic applications with good photocatalytic activity as well as magnetic recoverability.
Fig. 2 A shows a series of absorption spectra of the MB solution before and after mixing AF MCs into the aqueous solution of MB and placing the mixture under visible-light irradiation for various time periods without using any sacrificial reagents. As the irradiation time increases, the decomposition of the MB dye progressed gradually, and the decomposition was completed within 20 min. This result suggests that the MB dye was effectively decomposed under visible light with the assistance of the as-synthesized AF MCs. For comparison, the decomposition of MB with commercial TiO2 P25 and Ag–AgCl nanoparticles (NPs) (prepared according to Ref. 6) as reference photocatalysts, and without catalyst, was also investigated under identical conditions. As displayed in Fig. 2 B, the as-prepared AF MCs display an obviously superior catalytic performance over TiO2 P25, which could be explained by the weak adsorption of MB (2%) onto TiO2 P25 and near no absorption in the visible region of TiO2 P25. The as-prepared AF MCs also show a higher photocatalytic property than the visible light-driven photocatalysts Ag–AgCl NPs, which might be due to the higher charged anions (PO43−), leading to the stronger photocatalytic ability, as reported previously.21 When there was no catalyst in solution, no obvious change was observed in the UV-visible spectrum of the MB solution. Therefore, we may conclude that the as-prepared AF MCs possess a highly efficient photocatalytic activity under visible light.
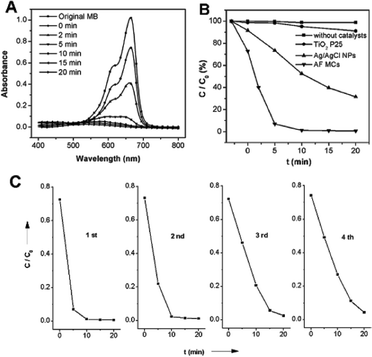 | ||
| Fig. 2 (A) UV-Visible spectra of the MB solution illuminated by visible light at different times with the assistance of AF MCs. (B) Photodecomposition of MB dye in solution over AF MCs, TiO2 P25, Ag–AgCl NPs and without catalyst under visible-light irradiation. (C) Irradiation-time dependence of the relative concentration C/C0 of MB in solution over AF MCs during repeated photooxidation experiments under visible light. | ||
We found that the photocatalysts turned dark when the photocatalytic reaction was completed, indicating the formation of Ag0 species through the reduction of Ag3PO4 by photo-induced electrons. A similar phenomenon was previously observed in a AgBr/SiO2 photolysis system, where the Ag metal was generated in the initial reaction stage and AgBr was not destroyed increasingly under successive UV irradiation.22 It has been reported that the metallic Ag could trap photo-induced electrons, and thus promote charge separation in contact with a photoexcited semiconductor,23 which consequently inhibited the reduction of Ag salts and made the hybrid composites stable under illumination.5–7,24–26 To evaluate whether the AF MCs were active and stable after partial Ag3PO4 was converted to Ag0 species, we further examined the recyclability of the AF MCs, which was also very important from a practical application point of view. Fig. 2 C plots the kinetic curves for the degradation of different MB dyes by using the same batch of AF MCs. In this case, after MB was completely decomposed, the AF MCs were collected by an external magnet and used for the next photocatalytic reaction (Fig. S4†). The catalytic efficiency of the AF MCs retains over 95% of its initial activity after at least four consecutive uses, suggesting the highly recyclable ability of the AF MCs. The XRD patterns of the AF MCs after each of the consecutive photocatalytic experiments are listed in Fig. S5.† It can be seen that a new peak corresponding to Ag0 appeared together with the XRD peaks of Ag3PO4 and Fe3O4 after the first cycle of the reaction, whereas no obvious change was observed after the reaction was repeated for another three times. This implies that the Ag0 formation initially occurred on the AF MCs, which might not continuously occur in the consecutive recycling reactions. In addition, no significant loss of photocatalytic efficiency was observed after four cycles of the reaction (Fig. 2 C). These results further demonstrate that the prepared AF MCs were efficient photocatalysts and could be repeatedly used in the photocatalytic process with the advantage of an easy separation with an external magnet.
We consider that when the as-prepared Fe3O4–Ag3PO4 photocatalyst is irradiated by visible light, a number of electron–hole pairs are generated. The photogenerated electrons are preferably transferred to the Ag+,26 and then the Ag metals that have been formed on the surface, which can trap the photoexcited electrons and thus inhibit the further decomposition of Ag3PO4. The self-stability mechanism makes the hybrid composites stable under visible light illumination (Fig. S6†). In general, the photogenerated holes can oxidize the MB dye directly. At the same time, the photogenerated electrons are expected to be trapped by O2 dissolved in the solution to form the reactive oxygen species1,5,27 that could also chemically oxidize the MB dye.
We further investigated the photocatalytic performance of the prepared AF MCs under natural sunlight. The absorption spectra of the solution at different illumination times are displayed in Fig. 3. The MB dye was completely decomposed within 10 min, indicating that the AF MCs could efficiently harvest sunlight to drive the catalytic reaction. Interestingly, we found that the photocatalytic activity of the AF MCs was higher under sunlight than under a lamp, based on the fact that it required a shorter time for MB decomposition under the former conditions. The enhanced activity might be elucidated by the broad band of the sunlight, including high-energy UV light.6
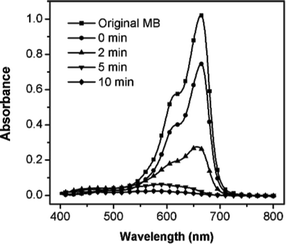 | ||
| Fig. 3 UV-Visible spectra of the MB solution before and after exposure to sunlight for different lengths of time with the assistance of the AF MCs. | ||
Conclusions
A simple and fast method has been demonstrated for the synthesis of magnetically separable AF MCs that can be well dispersed in water and easily recovered by an external magnet. The as-synthesized AF MCs exhibit a high adsorption ability toward MB dye, a strong absorption in the visible region, and a high efficiency and good recyclability for catalyzing the decomposition of MB dye under visible light and sunlight irradiation without any sacrificial reagents in the solution. It is believed that the easy preparation and efficient photocatalytic activity, in combination with the convenient recovery by an external magnet and high recyclability, of this type of nanophotocatalyst makes them substantially promising for practical applications in the degradation of organic pollutants for environmental remediation by the direct use of solar energy.Acknowledgements
This work was financially supported by the NSF of China (grant no.s 20975104, 21127901, and 20935005), the National Basic Research Program of China (973 Program, 2010CB933502), and the Chinese Academy of Sciences (KJCX2-YW-W25 and Y2010015).References
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- M. A. Fox and M. T. Dulay, Chem. Rev., 1993, 93, 341–357 CrossRef CAS.
- S. Ito, K. R. Thampi, P. Comte, P. Liska and M. Gratzel, Chem. Commun., 2005, 268–270 RSC.
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai, J. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931–7933 CrossRef CAS.
- C. An, S. Peng and Y. Sun, Adv. Mater., 2010, 22, 2570–2574 CrossRef CAS.
- L. Han, P. Wang, C. Zhu, Y. Zhai and S. Dong, Nanoscale, 2011, 3, 2931–2935 RSC.
- Z. Yi, J. Ye, N. Kikugawa, T. Kako, S. Ouyang, H. Stuart-Williams, H. Yang, J. Cao, W. Luo and Z. Li, Nat. Mater., 2010, 9, 559–564 CrossRef CAS.
- Y. Bi, S. Ouyang, N. Umezawa, J. Cao and J. Ye, J. Am. Chem. Soc., 2011, 133, 6490–6492 CrossRef CAS.
- Y. Bi, H. Hu, S. Ouyang, G. Lu, J. Cao and J. Ye, Chem. Commun., 2012, 48, 3748–3750 RSC.
- N. Umezawa, O. Shuxin and J. Ye, Phys. Rev. B, 2011, 83, 035202 CrossRef.
- C. T. Dinh, T. D. Nguyen, F. Kleitz and T. O. Do, Chem. Commun., 2011, 47, 7797–7799 RSC.
- P. V. Kamat, Chem. Rev., 1993, 93, 267–300 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- M. Zhu and G. Diao, J. Phys. Chem. C, 2011, 115, 18923–18934 CAS.
- V. Salgueiriño-Maceira, M. A. Correa-Duarte, M. Spasova, L. M. Liz-Marzán and M. Farle, Adv. Funct. Mater., 2006, 16, 509–514 CrossRef.
- S. Guo, S. Dong and E. Wang, Chem.–Eur. J., 2009, 15, 2416–2424 CrossRef CAS.
- L. W. Zhang, H. B. Fu and Y. F. Zhu, Adv. Funct. Mater., 2008, 18, 2180–2189 CrossRef CAS.
- H. Zhang, X. Lv, Y. Li, Y. Wang and J. Li, ACS Nano, 2009, 4, 380–386 CrossRef.
- M. Zhu, P. Chen and M. Liu, ACS Nano, 2011, 5, 4529–4536 CrossRef CAS.
- H. Huang, X. Li, Z. Kang, Y. Liu, H. Li, X. He, S. Lian, J. Liu and S. T. Lee, Dalton Trans., 2010, 39, 10593–10597 RSC.
- N. Kakuta, N. Goto, H. Ohkita and T. Mizushima, J. Phys. Chem. B, 1999, 103, 5917–5919 CrossRef CAS.
- T. Hirakawa and P. V. Kamat, Langmuir, 2004, 20, 5645–5647 CrossRef CAS.
- P. Wang, B. Huang, Q. Zhang, X. Zhang, X. Qin, Y. Dai, J. Zhan, J. Yu, H. Liu and Z. Lou, Chem.–Eur. J., 2010, 16, 10042–10047 CrossRef CAS.
- P. Wang, B. Huang, Z. Lou, X. Zhang, X. Qin, Y. Dai, Z. Zheng and X. Wang, Chem.–Eur. J., 2010, 16, 538–544 CrossRef CAS.
- X. Wang, S. Li, H. Yu, J. Yu and S. Liu, Chem.–Eur. J., 2011, 17, 7777–7780 CrossRef CAS.
- S. S. Soni, M. J. Henderson, J. F. Bardeau and A. Gibaud, Adv. Mater., 2008, 20, 1493–1498 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available. See DOI: 10.1039/c2ra20504a/ |
| This journal is © The Royal Society of Chemistry 2012 |
