Fluorescent organic–inorganic hybrid polyphosphazene microspheres for the trace detection of nitroaromatic explosives
Wei
Wei
a,
Xiaobin
Huang
*a,
Kuiyong
Chen
a,
Yiming
Tao
b and
Xiaozhen
Tang
ac
aSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai, 200240, People's Republic of China. E-mail: xbhuang@sjtu.edu.cn; Fax: +86-21-54741297; Tel: +86-21-54743264
bSchool of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai, 200240, People's Republic of China
cNational Key Laboratory of Metallic Matrix Composite Material, Shanghai Jiao Tong University, Shanghai, 200240, People's Republic of China
First published on 16th February 2012
Abstract
Highly cross-linked, intrinsically fluorescent organic–inorganic hybrid polymer microspheres bearing primary amine groups on the surface have been successfully prepared through a one-pot polycondensation of hexachlorocyclotriphosphazene with benzidine. Just by the single-step introduction of plentiful π-conjugated benzidine units in the structure, the resulting microspheres easily obtain both the luminescence and abundant active amino groups, with no need for more modification. Thus further using the microspheres as fluorescence-based nitroaromatic sensor, 2,4,6-trinitrotoluene, 2,4-dinitrotoluene, and picric acid can be effectively and sensitively detected. Because the nitroaromatic analytes can be effectively enriched on the surface of the microspheres by the charge-transfer complexing interaction between electron-deficient aromatic rings and electron-rich amino groups, which facilitates the electron transfer and energy transfer from microspheres to nitroaromatics, and finally leads to a significant and sensitive fluorescence quenching response. Moreover, the microspheres also exhibit remarkable thermal stability, photobleaching stability, and solvent resistance and dispersion ability in various solvents including both aqueous and organic media, owing to the highly cross-linked and organic–inorganic hybrid structure.
Introduction
Fast and reliable detection of trace explosives has aroused considerable attention during the past decade, owing to the globalization of an unprecedented severe terrorism menace.1–5 Nitroaromatic compounds such as 2,4,6-trinitrotoluene (TNT), 2,4-dinitrotoluene (DNT), and picric acid (PA) are common high explosives, the sensitive detection and identification of which have antiterrorism implications.6–8 Although various analytical techniques and strategies, including ion mobility spectrometry,9,10 gas chromatography,11,12 mass spectrometry,13,14 nuclear quadrupole resonance,15 energy dispersive X-ray diffraction,16 and surface enhanced Raman spectroscopy,17,18 have been reported for the highly sensitive detection of nitroaromatics, the requirement of bulky equipment, time-consuming procedures, and complicated manipulation are the bottle-necks for application of these methods to on-site field testing.19 As a more convenient and cost-effective alternative approach, fluorescence-quenching-based chemical detection has been widely employed in recent years, the main working mechanism of which is the electron transfer from electron-rich fluorophores to electron-deficient nitro compounds resulting in fluorescence quenching.20 Up to now, conjugated polymers,21,22 organic fluorescent molecules,23,24 and quantum dots25,26 have usually been developed as active materials for fluorescence-based nitroaromatic sensors.To achieve a high detection sensitivity and efficiency, it is essential for the target analytes to be enriched around the surface of fluorescent sensory materials via physical adsorption or chemical interaction, because the efficiency of fluorescence quenching is usually dependent on the adsorptive affinity of sensors to nitroaromatic molecules.27 It has been known that electron-rich primary amine groups can strongly bind electron-deficient nitroaromatics by the formation of acid–base pairing interactions and Meisenheimer complexes.8 In this regard, organic amines are always introduced to the sensory system as the receptors of nitroaromatic compounds, which can effectively enrich the nitroaromatic species around fluorophores, and greatly enhance the chemosensory efficiency. Zhang and co-workers recently reported a series of amine-modified fluorescence-based nitroaromatic sensors: amine-capped ZnS-Mn2+ nanocrystals,27 amine and dye molecule-modified silica nanoparticles,28 and inverted opal silica film with a hybrid monolayer of amino ligands and dye molecules.29 Though they all exhibit ultrasensitive fluorescence quenching response to trace nitroaromatics profiting from the enriching effect of amino groups, the combination of organic amino ligands and fluorophores is multistep, and the preparation procedures are complicated. Hence one-step fabrication of the fluorescence-based nitroaromatic sensor with primary amine groups is a key challenge and a continuous demand.
In the study reported herein, we prepare a new fluorescence-based nitroaromatic sensor containing active amino groups, that is poly(cyclotriphosphazene-co-benzidine) microspheres (PCPB-MS), just by a simple and one-pot method. The PCPB-MS with the highly cross-linked organic–inorganic hybrid structure, possesses not only the intrinsically fluorescent property, but also the abundant active amino groups on the surface, which can be easily achieved through the one-step polymerization between hexachlorocyclotriphosphazene (HCCP) and benzidine. The microspheres have both the fluorophores and organic amino ligands in the structure, and can be used directly for the detection of nitroaromatics without further modification. The primary amine groups on the surface of PCPB-MS can effectively enrich nitroaromatic species around the fluorophores, which facilitates the electron transfer and energy transfer from PCPB-MS to nitroaromatics, and results in an outstanding fluorescence quenching efficiency and detection sensitivity. In addition, the PCPB-MS also exhibits superior thermal stability, excellent photobleaching stability, and remarkable solvent resistance and dispersion ability in various solvents including both aqueous and organic media, owing to the highly cross-linked and organic–inorganic hybrid structure. Therefore it is promising for the PCPB-MS to be applied in the trace detection of nitroaromatic explosives.
Experimental
Materials
HCCP (synthesized as described in the literature30) was recrystallized from dry hexane followed by sublimation (60 °C, 0.05 mmHg) twice prior to use. Benzidine (99.5% purity) was obtained from Yuanhang Reagent Factory, Shanghai Yuming Industrial Co. Ltd. (Shanghai, China) and used directly. Triethylamine (TEA, AR), acetonitrile (AR), acetone (AR), and methanol (AR) were purchased from Sinopharm Chemical Regent Co. Ltd. (Shanghai, China) and used without further purification. TNT (AR) and DNT (99% purity) were used as received from Aladdin Chemistry Co. Ltd. (Shanghai, China). PA (99.8% purity) was supplied by Shantou Xilong Chemical Factory (Guangdong, China).Caution: Nitroaromatics such as TNT, DNT and PA are highly explosive and should be used with extreme caution.
Preparation of PCPB-MS
The facile preparation of PCPB-MS with active amino groups was carried out by a one-pot method: 2 mL of TEA was added to a round-bottomed flask containing 0.100 g of HCCP, 0.212 g of benzidine and 30 mL of acetonitrile. The molar ratio of benzidine to HCCP was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (benzidine was in excess over HCCP). The reaction was conducted in the darkness under ultrasonic irradiation (100 W, 40 kHz) at 45 °C for 48 h. The whole process was under the protection of nitrogen. After reaction, the precipitated product was isolated by centrifugation, washed three times using acetone and deionized water, respectively, and then dried in vacuum at 65 °C to yield PCPB-MS as a yellow powder.
1 (benzidine was in excess over HCCP). The reaction was conducted in the darkness under ultrasonic irradiation (100 W, 40 kHz) at 45 °C for 48 h. The whole process was under the protection of nitrogen. After reaction, the precipitated product was isolated by centrifugation, washed three times using acetone and deionized water, respectively, and then dried in vacuum at 65 °C to yield PCPB-MS as a yellow powder.
As a control sample, PCPB-MS with fewer free primary amine groups was also prepared according to the aforementioned reaction step, but benzidine was not in excess and the molar ratio of benzidine to HCCP equalled to their stoichiometric ratio, which was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Characterization
The field-emission scanning electron microscopy (FE-SEM) images were taken using a JSM-7401F field-emission scanning electron microscope (JEOL Ltd., Japan) at an activation voltage of 5 kV. The Fourier-transform infrared (FT-IR) measurements were conducted on a Spectrum 100 FT-IR spectrometer (Perkin-Elmer, Inc., USA) at room temperature (25 °C). The elemental analyses (EA) were performed using a Perkin-Elmer 2400-II instrument (Perkin-Elmer, Inc., USA). The thermogravimetric analyses (TGA) were performed on a Q5000IR thermogravimetric analyzer (TA Instruments, USA) in the temperature range from ambient temperature to 800 °C at a heating rate of 20 °C min−1 under nitrogen atmosphere. The ultraviolet-visible (UV-Vis) absorbance spectra were recorded with a Lambda 20 UV-Vis spectrophotometer (Perkin-Elmer, Inc., USA). The fluorescence emission spectra were measured by a QM/TM/IM steady-state & time-resolved fluorescence spectrofluorometer (PTI Ltd., USA) under excitation at 291 nm.Fluorescence quenching detection of nitroaromatics with PCPB-MS
Three typical nitroaromatics – TNT, DNT and PA – were selected as analytes, the detection of which was carried out through a series of spectrofluorometric titrations using the PCPB-MS as the probe. For each analyte, the typical test procedure is as follows: 2 mL of the methanol dispersion of PCPB-MS with concentration of 30 μg mL−1 was injected to a spectrophotometer quartz cuvette. In the absence of analyte, the fluorescence spectrum of pure PCPB-MS was first recorded. Subsequently, different amounts of analyte were respectively added into the above cuvette. Each time after fully mixing the analyte with microsphere dispersion, the fluorescence spectrum was collected at once. For comparison, the fluorescence responses of the PCPB-MS with fewer free primary amine groups to the selected nitroaromatics were also tested according to the same process.Results and discussion
Structure and morphology characterization
The single-step synthesis route of PCPB-MS is depicted in Scheme 1. In the solution of acetonitrile, PCPB-MS was efficiently prepared by the polymerization between HCCP and excess benzidine with TEA as an acid acceptor, which was, in essence, the nucleophilic replacement reaction between the terminal amino groups and P–Cl bonds. The formation process of the microspheres obeyed an oligomeric species absorbing mechanism,31 which was similar to our previously reported polyphosphazene microspheres.32,33 Obviously, the preparation procedure is simple, facile, technology-free, and one-pot.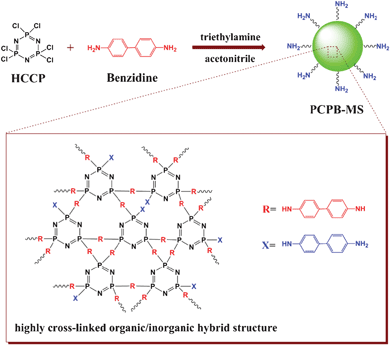 | ||
| Scheme 1 Illustration of the synthetic route and suggested chemical structure of PCPB-MS. | ||
The morphology of as-prepared PCPB-MS was investigated by FE-SEM. Fig. 1(A)–(D) show, with increasing magnification, that the PCPB-MS is almost monodispersed with perfect spherical shape and diameter about 2.3 μm on average. The chemical structure of the resulting microspheres was further characterized by FT-IR measurements. As shown in Fig. 2, the characteristic peak at 3343 cm−1 (a) is attributed to the N–H stretching vibration of –NH2, indicating the existence of primary amine groups on the PCPB-MS. The absorption at 3025 cm−1 (b) is assigned to the C–H stretching vibration of benzene ring. Phenyl absorption of benzidine units can be seen at 1612 cm−1 (c) and 1499 cm−1 (d). The absorptions at 1380 cm−1 (e) and 1263 cm−1 (f) correspond to the inter ring C–C band and C–N band of benzidine units, respectively. The stretching band at 1209 cm−1 (g) is attributed to the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N characteristic absorption of cyclotriphosphazene. Obviously, the peaks from (a) to (g) confirm that the products contain both the structure units of HCCP and benzidine. Furthermore, by comparison of the spectra of HCCP and PCPB-MS, it is found that the strong absorption peak of the P–Cl band of HCCP at 607 cm−1 (h) no longer exists after the polycondensation. Meanwhile, the characteristic peak of P–N band at 873 cm−1 (i) shows a little blue shift to 932 cm−1 (j) in the products, demonstrating the formation of new P–NH–(Ph) band. Thus we can reasonably assume the polycondensation between HCCP and benzidine has been successfully accomplished. The EA test gives the following results. Calc. for C42H36N10P3 (PCPB-MS): C, 65.2; H, 4.7; N, 18.1; P, 12.0%. Found: C, 65.8; H, 4.8; N, 18.0; P, 11.2%. Based on FT-IR and EA data, a possible cross-linked organic–inorganic hybrid and active amino-containing structure of PCPB-MS is proposed, as shown in Scheme 1.
N characteristic absorption of cyclotriphosphazene. Obviously, the peaks from (a) to (g) confirm that the products contain both the structure units of HCCP and benzidine. Furthermore, by comparison of the spectra of HCCP and PCPB-MS, it is found that the strong absorption peak of the P–Cl band of HCCP at 607 cm−1 (h) no longer exists after the polycondensation. Meanwhile, the characteristic peak of P–N band at 873 cm−1 (i) shows a little blue shift to 932 cm−1 (j) in the products, demonstrating the formation of new P–NH–(Ph) band. Thus we can reasonably assume the polycondensation between HCCP and benzidine has been successfully accomplished. The EA test gives the following results. Calc. for C42H36N10P3 (PCPB-MS): C, 65.2; H, 4.7; N, 18.1; P, 12.0%. Found: C, 65.8; H, 4.8; N, 18.0; P, 11.2%. Based on FT-IR and EA data, a possible cross-linked organic–inorganic hybrid and active amino-containing structure of PCPB-MS is proposed, as shown in Scheme 1.
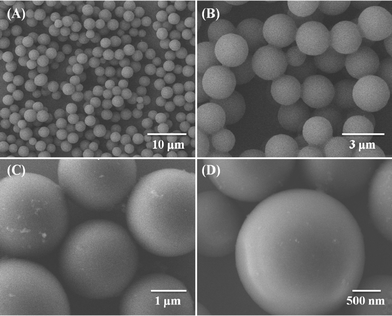 | ||
| Fig. 1 SEM images of PCPB-MS with different magnification. | ||
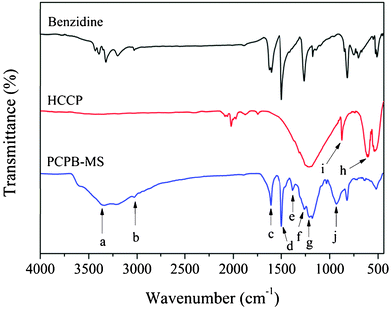 | ||
| Fig. 2 FT-IR spectra of benzidine, HCCP, and PCPB-MS. | ||
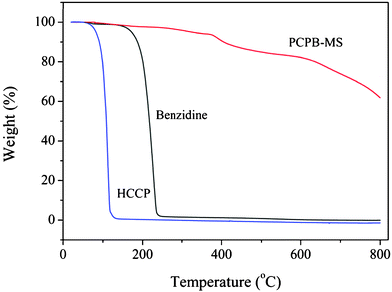
The thermal stability of PCPB-MS was also examined by TGA analysis, as displayed in Fig. 3. It is clear that PCPB-MS has much superior thermal stability compared with the two monomers, which may be owed to the highly cross-linked organic–inorganic hybrid structure.
Fluorescence emission of PCPB-MS for quenching detection of nitroaromatics
The as-prepared polyphosphazene microspheres with active amino groups exhibited an outstanding intrinsically luminescent feature, the UV absorption and fluorescence emission spectra of which dispersed in methanol are shown in Fig. 4(A). As can be seen, PCPB-MS displays a fluorescence emission peak at 387 nm when excited with 291 nm light, which is almost identical to the characteristic fluorescence emission peak of benzidine excited at 320 nm, indicating the luminescent feature of PCPB-MS is mainly contributed by the π-conjugated system of benzidine units introduced in the polymer structure. As is well-known, organic fluorescent dyes are always easily photobleached in the presence of oxygen, which to a great extent restricts their applications.34 In this study, it is found the PCPB-MS possesses excellent photobleaching stability, as shown in Fig. 4(B). The intensity of fluorescence emission peak is extremely stable to UV irradiation at 365 nm, implying the highly cross-linked structure can effectively retard photobleaching. In addition, the cross-linked and organic–inorganic hybrid structure also endows the microspheres with remarkable solvent resistance and dispersion ability in various solvents including both aqueous and organic media. Such advantages are the base for the further application of PCPB-MS in nitroaromatic detection.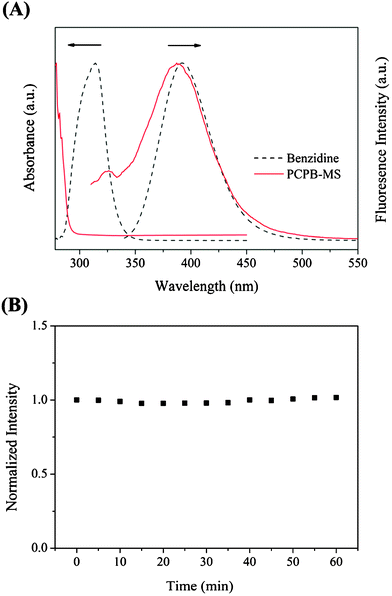 | ||
| Fig. 4 (A) UV absorption and fluorescence emission spectra of benzidine and PCPB-MS in methanol. (B) Normalized intensity of the fluorescence emission peak of PCPB-MS versus UV irradiation time (365 nm). | ||
Fig. 5 shows the fluorescence spectral response of PCPB-MS to the explosive analytes: TNT (A), DNT (B), and PA (C). All the three nitroaromatics exhibit effective quenching of the fluorescence of PCPB-MS. However, the order of quenching efficiency is PA ≫ DNT > TNT. To achieve almost full fluorescence quenching, 150 μg mL−1 of TNT or 100 μg mL−1 of DNT are needed. But for PA, just 20 μg mL−1 is enough. Obviously, PA is the strongest quencher of PCPB-MS fluorescence. The detectable minimum concentration of the three analytes is listed in Table 1. The detection sensitivity and efficiency for PA and TNT using PCPB-MS as the probe in this study are much superior to the previously reported fluorescent inorganic–organic hybrid polymer probe.35
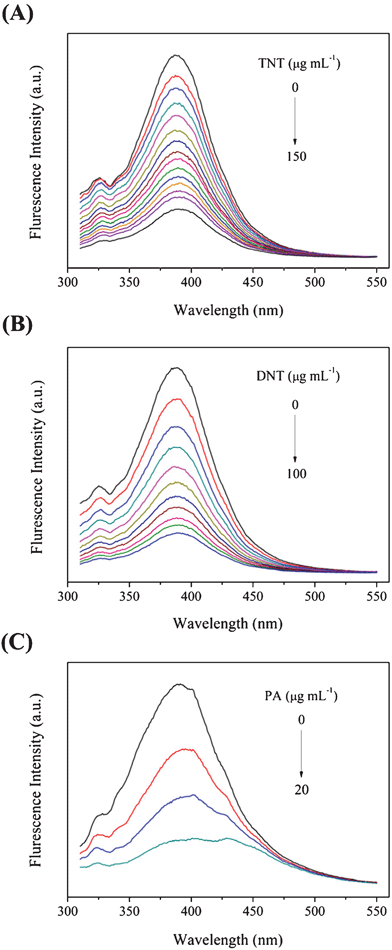 | ||
| Fig. 5 Fluorescence spectra of PCPB-MS dispersed in methanol (30 μg mL−1) with the addition of different amounts of (A) TNT (0–150 μg mL−1), (B) DNT (0–100 μg mL−1), and (C) PA (0–20 μg mL−1). | ||
| Analytes | |||
|---|---|---|---|
| TNT | DNT | PA | |
| Detectable concentration (μg mL−1) | 0.5 | 0.25 | 0.05 |
| K SV (M−1) | 2118 | 3647 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 134 |
In order to further quantify the quenching efficiency for the three selected nitroaromatics, Stern–Volmer equation was used as follows:
| (I0/I) − 1 = KSV[A] | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 134 M−1, which is 18 and 10-fold that of TNT and DNT, respectively, indicating its surprising superquenching efficiency to the photoluminescence of PCPB-MS. Such a value is even much higher than the reported quenching constant for PA using amine-capped ZnS-Mn2+ nanocrystals as the probe (34
134 M−1, which is 18 and 10-fold that of TNT and DNT, respectively, indicating its surprising superquenching efficiency to the photoluminescence of PCPB-MS. Such a value is even much higher than the reported quenching constant for PA using amine-capped ZnS-Mn2+ nanocrystals as the probe (34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870 M−1).27
870 M−1).27
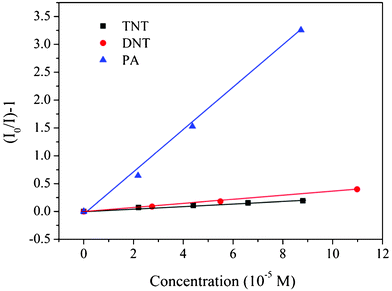 | ||
| Fig. 6 Stern–Volmer plots from PCPB-MS with the three nitroaromatic analytes. The concentration of PCPB-MS is 30 μg mL−1 in methanol. | ||
Mechanism for the fluorescence quenching response of PCPB-MS to nitroaromatics
Primary amines, such as 3-aminopropyltriethoxysilane and cysteamine, have been confirmed to have the ability to strongly bind TNT by the formation of acid–base pairing interaction and Meisenheimer complexes between the electron-rich amino group and the electron-deficient aromatic ring, which is reflected in the color change of the TNT solution from colorless to deep red when mixed with primary amines.27–29,36 In this study, a similar interaction between DNT and benzidine was observed and demonstrated by the UV-Vis absorbance spectra. As shown in Fig. 7(A), after mixing the methanol solution of DNT with the methanol solution of benzidine, the final solution becomes brownish red from colorless, and the absorption spectrum of which displays a remarkable absorption between 325 nm and 400 nm, indicating the occurrence of the strong molecular interaction between the amino group of benzidine and the aromatic ring of DNT. When the methanol solution of DNT was mixed the methanol dispersion of PCPB-MS, Fig. 7(B) shows also the increased absorption between 300 nm and 350 nm, together with the color change from colorless to light red. These observations reveal clearly that there are abundant active amino groups on the surface of PCPB-MS, and the PCPB-MS with amino groups can effectively enrich DNT species from solution through the charge-transfer interactions occurring between amino groups and DNT. Because DNT is a much weaker Lewis acid or electron acceptor than TNT and PA molecules,28 it is therefore reasonable to believe that TNT and PA can also be strongly bound onto the surface of PCPB-MS.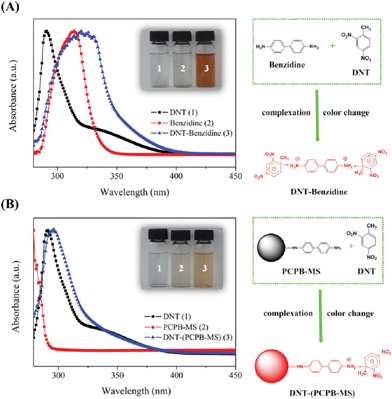 | ||
| Fig. 7 (A) UV-Vis absorbance spectra of DNT, benzidine, and DNT-benzidine mixture in methanol (inset colored image shows their corresponding colors). The right side illustrates the charge-transfer complexing interaction between benzidine and DNT molecules. (B) UV-Vis absorbance spectra of DNT, PCPB-MS, and DNT-(PCPB-MS) mixture in methanol (inset colored image shows their corresponding colors). The right side illustrates the charge-transfer complexing interaction between PCPB-MS and DNT molecules. | ||
Generally, the main mechanism of fluorescence quenching detection of nitroaromatic explosives is electron transfer from the excited electron-rich fluorophores to the electron-deficient nitroaromatics with relatively low LUMO energies.20,35 In addition, if the fluorophores and analytes are spatially close to each other, meanwhile the absorption band of analytes has an effective spectral overlapping with the emission band of fluorophores, then the resonance energy transfer from fluorophores to the non-emissive analytes will occur, which can dramatically amplify the fluorescence quenching efficiency and enhance the detection sensitivity.28 The efficiency of the resonance energy transfer is mainly up to the degree of spectral overlap between the absorption of analyte and the emission of fluorophore. Fig. 8 shows the normalized absorption spectra of TNT, DNT, and PA, plotted together with the normalized emission spectrum of PCPB-MS. As can be seen, almost no spectral overlap is observed for TNT, and DNT only exhibits a partial spectral overlap with PCPB-MS. However, the absorption of PA largely overlaps with the emission of PCPB-MS. The order of the degree of spectral overlap is: PA ≫ DNT > TNT. Hence in this study, the main mechanism for the fluorescence quenching response of PCPB-MS to TNT and DNT is electron transfer. The excited-state electrons of the benzidine units in the structure of PCPB-MS may transfer to the LUMO of TNT and DNT bound onto the surface of PCPB-MS, resulting in the effective fluorescence quenching. For PA, the fluorescence quenching process predominantly obeys resonance energy transfer mechanism besides common electron transfer. As illustrated in Scheme 2, based on the spatial proximity of PA molecules and benzidine units at the surface of PCPB-MS achieved by the formation of PA-amine complex, and also the large spectral overlap between the absorption of PA and the emission of PCPB-MS, the energy transfer from the excited state of the benzidine units to the ground state of PA may occur owing to the polar–polar resonance of donor and acceptor. Such coupling transitions can lead to the greatly amplified quenching of PCPB-MS fluorescence. Now it is easy to understand the discrepancy of detection efficiency among the three nitroaromatics.
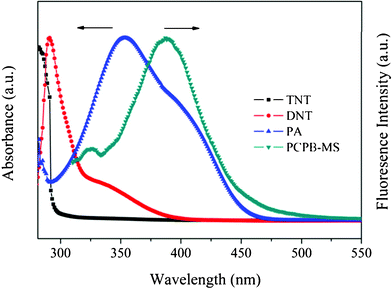 | ||
| Fig. 8 Normalized absorption spectra of TNT, DNT, and PA plotted together with the normalized emission spectrum of PCPB-MS in methanol. | ||
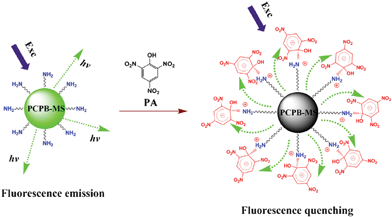 | ||
| Scheme 2 Schematic illustration of the fluorescence quenching detection of PA with PCPB-MS based on resonance energy transfer mechanism. | ||
Fig. 9 further compares the spectral response of the –NH2-containing PCPB-MS with that of the PCPB-MS with less –NH2 to PA. It is clearly detected that the fluorescence quenching of the –NH2-containing PCPB-MS is quite large compared to that of the PCPB-MS with less –NH2 in the presence of 10 μg mL−1 PA, demonstrating the remarkable enhancement of fluorescence quenching efficiency by the primary amine ligands. In the case of the –NH2-containing PCPB-MS, the electron-rich amino groups can effectively absorb the electron-deficient PA molecules onto the surface of the microspheres. Thus compared with the PCPB-MS with less –NH2, the –NH2-containing PCPB-MS possesses a much higher affinity to the PA molecules, which may facilitate the electron transfer and energy transfer, and finally leads to a higher quenching efficiency and sensitivity.
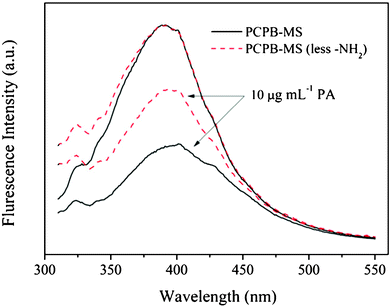 | ||
| Fig. 9 Comparison of fluorescence spectral response to PA of PCPB-MS with that of the PCPB-MS with less –NH2. | ||
Conclusions
In summary, highly cross-linked organic–inorganic hybrid polymer microspheres, PCPB-MS, are prepared by a one-step polycondensation between HCCP and benzidine. Due to the presence of plentiful π-conjugated benzidine units in the structure, the PCPB-MS has intrinsically fluorescent feature and abundant primary amine groups on the surface. Meanwhile, the cross-linked and organic–inorganic hybrid structure also endows the microspheres with remarkable thermal stability, photobleaching stability, and solvent resistance and dispersion ability in various solvents including both aqueous and organic media. The further application of PCPB-MS in the trace detection of nitroaromatic explosives demonstrates that nitroaromatics (TNT, DNT, and PA) can strongly quench the fluorescence of PCPB-MS. Especially for PA, a surprising superquenching efficiency is observed. It is because the nitroaromatic analytes can be effectively enriched on the surface of microspheres by the charge-transfer complexing interaction between electron-deficient aromatic rings and electron-rich amino groups that facilitates the electron transfer and energy transfer from microspheres to nitroaromatics, and finally leads to a significant and sensitive fluorescence quenching response. Thus it is promising for PCPB-MS to be used as a fluorescence-based nitroaromatic sensor.Acknowledgements
This work was supported by the foundation of Key Laboratory for Advanced Materials Processing Technology, Ministry of Education, P. R. China (No. 2010003), Shanghai Science & Technology Committee (No. 10ZR1416100) and Shanghai Leading Academic Discipline Project (No. B202).References
- A. Fainberg, Science, 1992, 255, 1531 CrossRef CAS.
- J. Yinon, Anal. Chem., 2003, 75, 98A CrossRef.
- S. Singh, J. Hazard. Mater., 2007, 144, 15 CrossRef CAS.
- L. Senesac and T. G. Thundat, Mater. Today, 2008, 11, 28 CrossRef CAS.
- J. S. Caygill, F. Davis and S. P. J. Higson, Talanta, 2012, 88, 14 CrossRef CAS.
- J. I. Steinfeld, Annu. Rev. Phys. Chem., 1998, 49, 203 CrossRef CAS.
- S. J. Toal and W. C. Trogler, J. Mater. Chem., 2006, 16, 2871 RSC.
- Y. Engel, R. Elnathan, A. Pevzner, G. Davidi, E. Flaxer and F. Patolsky, Angew. Chem., Int. Ed., 2010, 49, 6830 CrossRef CAS.
- D. D. Fetterolf and T. D. Clark, J. Forensic Sci., 1993, 38, 28 CAS.
- R. G. Ewing, D. A. Atkinson, G. A. Eiceman and G. J. Ewing, Talanta, 2001, 54, 515 CrossRef CAS.
- J. A. Caulfield, T. J. Bruno and K. E. Miller, J. Chem. Eng. Data, 2009, 54, 1814 Search PubMed.
- G. W. Cook, P. T. LaPuma, G. L. Hook and B. A. Eckenrode, J. Forensic Sci., 2010, 55, 1582 Search PubMed.
- R. G. Cooks, Z. Ouyang, Z. Takats and J. M. Wiseman, Science, 2006, 311, 1566 CrossRef CAS.
- Y. Zhang, X. Ma, S. Zhang, C. Yang, Z. Ouyang and X. Zhang, Analyst, 2009, 134, 176 RSC.
- A. N. Garroway, M. L. Buess, J. B. Miller, B. H. Suits, A. D. Hibbs, G. A. Barrall, R. Matthews and L. J. Burnett, IEEE Trans. Geosci. Remote Sens., 2001, 39, 1108 Search PubMed.
- R. D. Luggar, M. J. Farquharson, J. A. Horrocks and R. J. Lacey, X-Ray Spectrom., 1998, 27, 87 CAS.
- J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834 CrossRef CAS.
- A. Tao, F. Kim, C. Hess, J. Goldberger, R. He, Y. Sun, Y. Xia and P. Yang, Nano Lett., 2003, 3, 1229 CrossRef CAS.
- M. S. Meaney and V. L. McGuffin, Anal. Chim. Acta, 2008, 610, 57 CrossRef CAS.
- J. V. Goodpaster and V. L. McGuffin, Anal. Chem., 2001, 73, 2004 CrossRef CAS.
- S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef CAS.
- A. Narayanan, O. P. Varnavski, T. M. Swager and T. Goodson III, J. Phys. Chem. C, 2008, 112, 881 CrossRef CAS.
- K. J. Albert and D. R. Walt, Anal. Chem., 2000, 72, 1947 CrossRef CAS.
- A. Yildirim, H. Acar, T. S. Erkal, M. Bayindir and M. O. Guler, ACS Appl. Mater. Interfaces, 2011, 3, 4159 Search PubMed.
- R. Wilson, D. G. Spiller, I. A. Prior, R. Bhatt and A. Hutchinson, J. Mater. Chem., 2007, 17, 4400 RSC.
- G. H. Shi, Z. B. Shang, Y. Wang, W. J. Jin and T. C. Zhang, Spectrochim. Acta, Part A, 2008, 70, 247 CrossRef.
- R. Tu, B. Liu, Z. Wang, D. Gao, F. Wang, Q. Fang and Z. Zhang, Anal. Chem., 2008, 80, 3458 CrossRef CAS.
- D. Gao, Z. Wang, B. Liu, L. Ni, M. Wu and Z. Zhang, Anal. Chem., 2008, 80, 8545 CrossRef CAS.
- Q. Fang, J. Geng, B. Liu, D. Gao, F. Li, Z. Wang, G. Guan and Z. Zhang, Chem.–Eur. J., 2009, 15, 11507 CrossRef CAS.
- R. D. Jaeger and M. Gleria, Prog. Polym. Sci., 1998, 23, 179 CrossRef CAS.
- S. Shim, S. Yang and S. Choe, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3967 CrossRef CAS.
- L. Zhu, Y. Zhu, Y. Pan, Y. Huang, X. Huang and X. Tang, Macromol. React. Eng., 2007, 1, 45 Search PubMed.
- W. Wei, X. Huang, X. Zhao, P. Zhang and X. Tang, Chem. Commun., 2010, 46, 487 RSC.
- L. Song, E. J. Hennink, I. T. Young and H. J. Tanke, Biophys. J., 1995, 68, 2588 CrossRef CAS.
- X. M. Hu, Q. Chen, D. Zhou, J. Cao, Y. J. He and B. H. Han, Polym. Chem., 2011, 2, 1124 RSC.
- D. Gao, Z. Zhang, M. Wu, C. Xie, G. Guan and D. Wang, J. Am. Chem. Soc., 2007, 129, 7859 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |