Transparent, flexible and luminescent composite films by incorporating CuInS2 based quantum dots into a cyanoethyl cellulose matrix†
Huiqing
Wang
a,
Ziqiang
Shao
*a,
Bingkun
Chen
ab,
Teng
Zhang
ab,
Feijun
Wang
a and
Haizheng
Zhong
*ab
aSchool of Materials Science &Engineering, Beijing Institute of Technology, 5 Zhongguancun South Street, Beijing, China. E-mail: shaoziqiang@263.net; Fax: +86-10-68941797; Tel: +86-10-68941797
bMicro Nano Research Center, Beijing Institute of Technology, 5 Zhongguancun South Street, Beijing, China. E-mail: hzzhong@bit.edu.cn
First published on 23rd January 2012
Abstract
Transparent luminescent composite films were fabricated by incorporating CuInS2 based quantum dots (QDs) into a cyanoethyl cellulose (CEC) matrix.
Quantum dots (QDs) have attracted considerable attentions for their size-dependent properties and potential applications in optical electronic devices.1 Hybrid composites, made by incorporating QDs into a polymer matrix, offer an attractive means to fabricate luminescent, transparent and flexible films for light-emitting and displaying applications.2 Although several works have highlighted the possibility of fabricating hybrid composite films with high photoluminescence (PL) and applying them in optical devices, the inherent incompatibility and reduction of the PL intensity are still great challenges in the process of incorporating QDs into polymer matrix.3 Here we report the fabrication of transparent and flexible hybrid composite films with tunable emission colors and high quantum yields (QYs) up to 30%.
Previous work on hybrid QDs and polymer composite films usually use QDs containing Cd and Pb elements, which are toxic materials.2,3 It is of great interest to look for alternative lower toxic and environmentally friendly materials. Recently, I-III-VI compounds such as CuInS2 and CuInSe2 appeared as possible low toxic alternative materials and much attention has been paid to their fabrication and properties.4 We recently achieved high quality CuInS2 based QDs with tunable emissions in the visible and near-infrared region.5 This provides a chance to apply CuInS2 based QDs as less toxic emitters in hybrid composite films for practical applications.
Cellulose is the most common and inexhaustible raw polymer material and can be modified into various derivatives with improved properties.6 Cyanoethyl cellulose (CEC) with a high degree of substitute value has a high dielectric constant and low dielectric loss factor induced by the strong electron-attracting cyanogen group.7 For their high dielectric constant and transparency, it is considered to be a suitable candidate as a matrix for light converters of light-emitting diodes (LEDs).8
The incorporation of luminescent QDs into a cellulose matrix has been previous studied.9 An obvious challenge is the choice of suitable solvent to combine the polymer and QDs. Herein we developed a new strategy to fabricate hybrid composite films by incorporating CuInS2 QDs into the CEC matrix. This method can produce transparent, flexible color-tunable composite films with PL QYs up to ∼30%. The key point of the successful preparation is the solvent selectivity of CEC and the surface modification of QDs. We further demonstrate a simple model pattern for displaying applications and studied the QD content effects on the composite films by using absorption and PL spectra.
CuInS2 QDs and polymer solutions were prepared individually and subsequently mixed according to their volume in order to form the QD–polymer mixtures. CEC derivatives are not soluble in toluene and chloroform, which are good solvents for oleylamine (OLA) capped CuInS2–ZnS QDs. N,N'-dimethylformamide (DMF) was found to be a suitable solvent of CEC, having a dielectric constant exceeding 15; however, CuInS2–ZnS QDs with OLA as ligands are not soluble in DMF. To make the QDs and CEC compatible in one solvent, the OLA capped CuInS2 QDs were transferred into DMF by partial ligand exchange with 3-mercaptopropionic acid (MPA) through a simple ultrasonication assisted method (see ESI†). Solutions of CuInS2 based QDs and CEC in DMF were mixed according to their volume to make approximately 2.5, 5, 10, and 25 wt% QD solutions. The mixed solutions were poured into glass Petri dishes. Then, the samples were left in the oven under vacuum at 60 °C for 24 h to completely evaporate the solvent. Finally, the films were carefully peeled from the glass surface. The films are flexible transparent, and have thicknesses on the order of ∼0.5 mm, depending on the amount of casting solution.
Fig. 1a shows the optical picture of CuInS2–ZnS/CEC nanocomposite films with 10 wt% QD content under daylight and UV 365 nm excitation. The films are clear, robust, with thicknesses of 0.05 to 0.5 mm depending on the concentration of CEC/QDs solution. Black words in the paper are seen clearly with the composite films under ambient visible light, demonstrating their good transparency. Under UV light excitation, the composite films exhibit a bright PL. Their luminescent color of the films can be tuned from green to yellow, orange, pink and red by varying CuInS2 based QDs (shown in Fig. 1b), which covered a wide wavelength region from 500 nm to 750 nm. This characteristic meets the color requirements in many light-emitting and displaying applications, including white LEDs that use the yellow fluorescent film by blue InGaN excitation.3–6
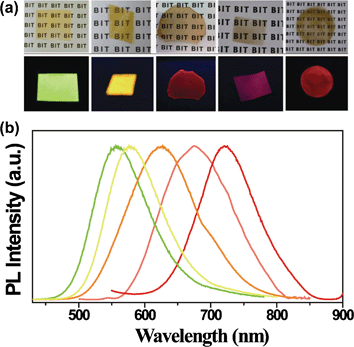 | ||
| Fig. 1 (a) Photographs of CEC films incorporated 10 wt% CuInS2 based QDs with different emission under visible light; (b) corresponding PL spectra of composite films. | ||
Because the composite films are flexible and luminescent, they are very good candidates for flexible fashionable displays. Similar to paper, the flexible films can be cut into different patterns. Fig. 2a shows a picture of hybrid composite films with rabbit shape under visible light. Under UV light excitation, the composite film exhibits red fluorescent under UV light in the dark, which is beautiful luminescent art. Besides, the ease in tailoring shapes, the composite films are also rollable. Fig. 2c shows an example of rolled films. This endows them potential applications portable displays, especial for fashion show.
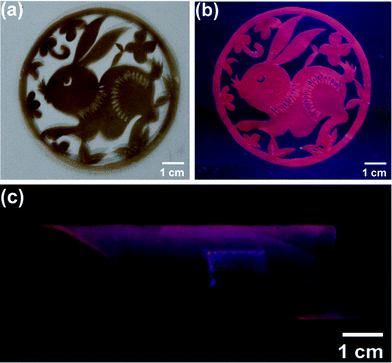 | ||
| Fig. 2 Photos of the nanocomposite films with rabbit-shape under visible light (a) and UV light (b), (c) rolled under UV light. | ||
The QD content in the hybrid composite films affects their transparency and PL properties. We systematically studied the influence of the QD content on the transparency through UV-vis absorption spectroscopic analysis. As shown in Fig. 3a, the pure CEC film has a 88 % transmittance at 700 nm, the hybrid composite film with 2.5 wt%, 5 wt%, and 10 wt% CuInS2-ZnS QDs content have a transmittance of 84, 79, 72 % at 700 nm, respectively. The composite film with QD content of 25 wt% dramatically decreased to 56 %.This can be explained by phase separation and particle aggregation, which was confirmed by optical images (data not shown) and AFM characterizations (see Fig. S1, ESI†). The QD content also affects the PL intensity of the films. Fig. 3b shows the PL intensity of the composite film with different QD content. It was observed that the composite films have a maximum PL intensity for the sample with 10 % QD content. The PL emission peak of the samples exhibits a red shift when the QD content increases, especially for the sample with 25 wt%. This may be due to self-absorption effects (the relative emission at short wavelength from small dots was absorbed by the larger ones), or due to the energy transfer in the QD aggregates (the exciton absorbed by small dots transfers to the larger ones and emits at a longer wavelength).10
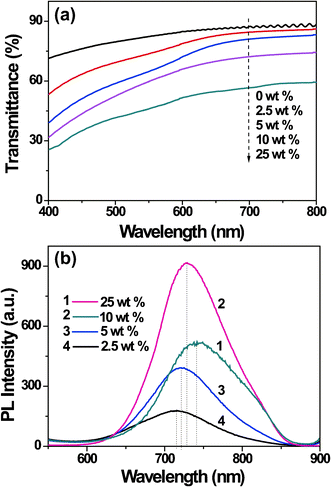 | ||
| Fig. 3 UV-vis spectra (a) and PL emission spectra (b) of hybrid composite films with QDs contents of 2.5 wt%, 5.0 wt%, 10 wt%, 25 wt% at a thickness of 0.5 mm. | ||
The incorporation of CuInS2 QDs into the CEC matrix enables the composite films’ luminescent characteristics. Their thermal properties can also be influenced by the CuInS2 QDs. Thermogravimetric analysis (TGA) was performed and the results are shown in Fig. S2, ESI.† The decomposition of the cellulose main chains was delayed from 256 °C to 307 °C after the addition of 10 wt% QDs. This reveals that the QDs-CEC composite film has better thermal stability than CEC alone.
Conclusions
In summary, transparent, flexible, fluorescent composite films based on low toxic CuInS2–ZnS QDs and CEC were successfully prepared via combining the surface modification of QDs and the casting method of the mixed solution. The films showed a high transparency and their emissive colour can be tunable over green, yellow, orange, pink and red by varying the QD content. Besides, the film is flexible and rollable for fashionable displays. These composite films have the potential to be employed in solid state lighting emission diodes, rollable displays, light-emitting devices and laser displays. It could also be important for developing other hybrid QDs and polymer composites.We gratefully acknowledge the financial support of the NFSC (Research Grants 51003005) and National Basic Research Program of China (No.2011CB933600). The authors thank Dr Wenjun Wang, Ms. Linlin Tan, Huijuan Liu for material preparation and helpful discussion.
References
-
(a) G. D. Scholes, Adv. Funct. Mater., 2008, 18, 1157 CrossRef CAS
; (b) D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2009, 110, 389 CrossRef
.
-
(a) J. Lee, V. C. Sundar, J. R. Heine, M. G. Bawendi and K. F. Jensen, Adv. Mater., 2000, 12, 1102 CrossRef CAS
; (b) H. Althues, R. Palkovits, A. Rumplecker, P. Simon, W. Sigle, M. Bredol, U. Kynast and S. Kaskel, Chem. Mater., 2006, 18, 1068 CrossRef CAS
; (c) G. Wegner, M. M. Demir, M. Faatz, K. Gorna, R. Munoz-Espi, B. Guillemet and F. Groehn, Macromol. Res., 2007, 15, 95 CrossRef CAS
; (d) H. Althues, J. Henle and S. Kaskel, Chem. Soc. Rev., 2007, 36, 1454 RSC
; (e) E. M. Likovich, R. Jaramillo, K. J. Russell, S. Ramanathan and V. Narayanamurti, Adv. Mater., 2011, 23, 4521 CrossRef CAS
.
-
(a) L. Pang, Y. M. Shen, K. Tetz and Y. Fainman, Opt. Express, 2005, 13, 44 CrossRef CAS
; (b) H. Zhang, C. Wang, M. Li, J. Zhang, G. Lu and B. Yang, Adv. Mater., 2005, 17, 853 CrossRef CAS
; (c) X. D. Cao, C. M. Li, H. Bao, Q. Bao and H. Dong, Chem. Mater., 2007, 19, 3773 CrossRef CAS
; (d) S. Yang, Q. Li, L. Chen and S. Chen, J. Mater. Chem., 2008, 18, 5599 RSC
; (e) H. Sun, H. Zhang, J. Ju, J. Zhang, G. Qian, C. Wang, B. Yang and Z. Y. Wang, Chem. Mater., 2008, 20, 6764 CrossRef CAS
; (f) H. Zhang, Y. Tang, J. Zhang, M. Li, X. Yao, X. Li and B. Yang, Soft Matter, 2009, 5, 4113 RSC
; (g) H. Wei, H. Sun, H. Zhang, C. Gao and B. Yang, Nano Res., 2010, 3, 496 CrossRef CAS
; (h) C. Woelfle and R. O. Claus, Nanotechnology, 2007, 18, 025402 CrossRef
.
- B. K. Chen, H. Z. Zhong and B. S. Zou, Prog. Chem., 2011, 23, 2276 CAS
.
-
(a) H. Z. Zhong, M. F. Ye, Y. J. He, J. P. Ye, C. He, C. H. Yang and Y. F. Li, Chem. Mater., 2008, 20, 6434 CrossRef CAS
; (b) H. Z. Zhong, S. S. Lo, T. Mirkovic, Y. C. Li, Y. Q. Ding, Y. F. Li and G. D. Scholes, ACS Nano, 2010, 4, 5253 CrossRef CAS
; (c) H. Z. Zhong, Z. B. Wang, E. Bovero, Z. H. Lu, F. C. J. M. van Veggel and G. D. Scholes, J. Phys. Chem. C, 2011, 115, 12396 CrossRef CAS
; (d) H. Z. Zhong, B. K. Chen and B. S. Zou, Chinese Patent, 2011, 201110259596.3 Search PubMed.
-
(a) J. L. Braun and J. F. Kadla, Biomacromolecules, 2005, 6, 152 CrossRef CAS
; (b) Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479 CrossRef CAS
.
-
(a) G. R. Saad, Polym. Int., 994, 34, 411 CrossRef
; (b) L. G. Wang and Y. Huang, Macromolecules, 2004, 37, 303 CrossRef CAS
.
-
(a)
H. Y. Mano. Yasuhiro, Japanese Patent, 1993, 5251181 Search PubMed
; (b) M. Karakawa, M. Chikamatsu, C. Vakamoto, Y. Maeda, S. Kubota and K. Yase, Macromol. Chem. Phys., 2007, 208, 2000 CrossRef CAS
; (c) Y. Okahisa, A. Yoshida, S. Miyaguchi and H. Yano, Compos. Sci. Technol., 2009, 69, 1958 CrossRef CAS
.
-
(a) T. Abitbol and D. Gray, Chem. Mater., 2007, 19, 4270 CrossRef CAS
; (b) T. Abitbol and D. G. Gray, Cellulose, 2009, 16, 319 CrossRef CAS
; (c) T. Abitbol, J. T. Wilson and D. G. Gray, J. Appl. Polym. Sci., 2011, 119, 803 CrossRef CAS
; (d) T. Niu, Y. Q. Gu and J. G. Huang, J. Mater. Chem., 2011, 21, 651 RSC
.
-
(a) M. V. Artemyev, U. Woggon, H. Jaschinski, L. I. Gurinovich and S. V. Gaponenko, J. Phys. Chem. B, 2000, 104, 11617 CrossRef CAS
; (b) C. R. Kagan, C. B. Murray, M. Nirmal and M. G. Bawendi, Phys. Rev. Lett., 1996, 76, 1517 CrossRef CAS
; (c) J. Lim, S. Jun, E. Jang, H. Baik, H. Kim and J. Cho, Adv. Mater., 2007, 19, 1927 CrossRef CAS
; (d) V. Rinnerbauer, H.-J. Egelhaaf, K. Hingerl, P. Zimmer, S. Werner, T. Warming, A. Hoffmann, M. Kovalenko, W. Heiss, G. Hesser and F. Schaffler, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 085322 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: materials, preparation, measurements, AFM images and TG curves. See DOI: 10.1039/c2ra01359b |
| This journal is © The Royal Society of Chemistry 2012 |
