Homochiral 1D-helical coordination polymers from achiral cucurbit[5]uril: hydroquinone-induced spontaneous resolution†
Kai
Chen
a,
Ying-Feng
Hu
b,
Xin
Xiao
a,
Sai-Feng
Xue
a,
Zhu
Tao
*a,
Yun-Qian
Zhang
a,
Qian-Jiang
Zhu
a and
Jing-Xin
Liu
*b
aKey Laboratory of Macrocyclic and Supramolecular Chemistry of Guizhou Province, Guizhou University, Guiyang, 550025, P. R. China. E-mail: gzutao@263.net (Z. Tao); Fax: +86-851-3620906
bCollege of Chemistry and Chemical Engineering, Anhui University of Technology, Maanshan, 243002, China. E-mail: jxliu411@ahut.edu.cn (J.-X. Liu); Fax: +86-555-2311552
First published on 6th March 2012
Abstract
Compared to a linear 1D coordination polymer of cucurbit[5] uril with Dy3+ ion, a pair of homochiral 1D-helical coordination polymers of cucurbit[5] uril with Dy3+ were obtained by spontaneous resolution upon crystallization in the presence of achiral hydroquinone, suggesting hydroquinone acts as an inducer of chiral helix.
As one of the most widespread phenomena in nature, chirality has already been the focus of much work.1–4 The interest is for theoretical and practical reasons. Firstly, chirality is of fundamental importance for understanding the origin of life and early evolution.1,2 Naturally occurring amino acids and sugars, which are basal building blocks of many biomacromolecules, such as proteins, enzymes and nucleic acids, adopt a one-handed helical conformation. However, the origin of this homochirality is not yet fully understood. Secondly, chirality plays a key role in both medicinal chemistry and material science.3–5 For example, R-thalidomide is an effective sedative, whereas S-thalidomide is a teratogen. However, pharmacists and doctors did not know this in the 1950s, which caused the famous thalidomide tragedy.6
Recently, much interest has been focused on the design and construction of chiral coordination polymers because of not only their intriguing architectures, which provide opportunities to study intriguing chiral topology, but also their potential applications in enantioselective separation,7 asymmetric catalysis,8 nonlinear optical9 and magnetic10 properties. Generally, there are two strategies to obtain chiral coordination polymers. The first approach is using enantiopure chiral species (chiral organic ligands or chiral metal complexes) or prochiral species, which yields enantiopure products. A number of chiral coordination polymers have been developed using this approach.7–11 The second method is using achiral ligands under spontaneous resolution without any chiral auxiliary, such as chiral catalyst and template. In a chirally unbiased world the crystallization of spontaneous resolution should generate right- or left-handed chiral crystals with equal probability, and the enantiomeric excess should be zero. A result of nonzero enantiomeric excess means the chiral symmetry is broken. To date, only a few examples of chiral symmetry breaking have been reported.12
Our research interest in this vein has been focused on the use of cucurbit[n]uril (n = 5–8, 10, hereafter abbreviated as Q[n]; Fig. 1), a class of achiral organic macrocyclic cavitand.13 Q[n] has been widely used to construct all kinds of host–guest complexes and coordination polymers.14 By contrast, examples of chiral coordination polymers based on Q[n] are still rather rare in the literature.15–18 Generally, Q[5] ligands can coordinate many kinds of metal cations to form isolated molecular capsules or 1D linear coordination polymers.19 However, our group recently found that in the presence of p-hydroxybenzoic acid, the Q[5] molecules are interlinked into unusual 2D networks.20 We hypothesize that small organic molecules may play important roles in constructing multidimensional Q[n]-based coordination polymers. So, some small organic molecules with aromatic groups were purposefully added in the reaction solution of Q[5] with lanthanide cations when constructing multidimensional Q[5]-based coordination polymers. In the present work we report a novel homochiral 1D-helical coordination polymer of achiral Q[5] with Dy3+ obtained by spontaneous resolution upon crystallization in the presence of achiral organic molecule hydroquinone (Hyq).
![Molecular structure of cucurbit[n]uril (n = 5–8, 10).](/image/article/2012/RA/c2ra01132h/c2ra01132h-f1.gif) | ||
| Fig. 1 Molecular structure of cucurbit[n]uril (n = 5–8, 10). | ||
Reaction of Q[5] with nitrate salts of dysprosium resulted in colorless crystals of [Dy(H2O)4Q[5]](NO3)2Cl·7H2O (compound 1, S1 in ESI). The single-crystal X-ray structure (S2 in ESI) of compound 1 reveals a 1D coordination polymer of alternating Q[5] building units and Dy3+ cations that extends along the c axis. The asymmetric unit of 1 consists of one Q[5] molecule, one Dy3+ cation, one nitrate anion, two chloride anions, and four coordinating and twelve non-coordinating water molecules. An ORTEP view of 1 is shown in Fig. 2, in which the Dy3+ ions are octacoordinated. Each Dy3+ ion features coordination by four water molecules, four carbonyl groups from two neighboring Q[5] molecules. The bond lengths of Dy–Ocarbonyl and Dy–Owater are 2.328(7)–2.391(7) Å and 2.333(8)–2.369(8) Å, respectively. Notably, each Q[5] molecule is coordinated by two dysprosium cations and each dysprosium cation is coordinated to two Q[5] molecules, leading to the formation of a 1D linear chain structure (Fig. 3).
![ORTEP diagram of compound 1 and illustration of the chiral environment around Dy1. The neighboring {Q[5]Dy(H2O)4Q[5]}3+ cations have different helical chirality. Displacement ellipsoids are drawn at the 30% probability level. H atoms, solvate water molecules and anions are omitted for clarity.](/image/article/2012/RA/c2ra01132h/c2ra01132h-f2.gif) | ||
| Fig. 2 ORTEP diagram of compound 1 and illustration of the chiral environment around Dy1. The neighboring {Q[5]Dy(H2O)4Q[5]}3+ cations have different helical chirality. Displacement ellipsoids are drawn at the 30% probability level. H atoms, solvate water molecules and anions are omitted for clarity. | ||
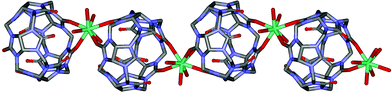 | ||
| Fig. 3 1D chain structure of compound 1. H atoms, solvate water molecules and anions are omitted for clarity. O = red, C = grey, N = light blue and Dy = blue. Symmetry codes: A x + 1/2, −y + 1/2, z + 1/2. | ||
In the presence of the organic molecule Hyq, reaction of Q[5] with nitrate salts of dysprosium resulted in red brown crystals of [Dy(H2O)4Q[5]](C6H6O2)(NO3)3·7H2O (compound 2; 2a and 2b are enantiomorphs; S1 in ESI). Single-crystal X-ray diffraction analyses (S2 in ESI) revealed that 2a and 2b crystallize in the Sohncke space group P61 (no. 169) and P65 (no. 170). The observed absolute structure (Flack) parameters of these two enantiomers are −0.001(10) and 0.020(18),21 respectively. Here we mainly describe the detailed crystal structure of 2a. At first glance, the coordination geometry of the Dy3+ ions in compound 2a is very similar to that in 1. In compound 2a, each Dy3+ ion is eight-coordinated by four water molecules and four carbonyl groups from two chelating Q[5] ligands. The Dy–Ocarbonyl and Dy–Owater distances are 2.316(3)–2.368(3) Å and 2.344(3)–2.371(3) Å, respectively, which are slightly different from those of their counterparts in 1. A closer inspection reveals that the coordination structures of these two compounds are inherently different. For these two compounds, each coordinated Q[5] may be regarded as a bidentate ligand to one Dy3+ ion center. In other words, each Dy3+ ion is coordinated by two bidentate ligands, which may be compared to the blades in a two-fold propeller, resulting in helical Δ/Λ chirality. In the case of compounds 2a and 2b, the neighboring metal centers have the same Δ- or Λ-configuration (Fig. 4), leading to a twist of the chain and the homochiral right- or left-handed helical structure finally forms. As can be seen in Fig. 5, each turn of the helix in compound 2 contains six units of Q[5] molecules and Dy3+ ions. This arrangement gives a pitch of 46.109(12) Å, which is the same as the c axis length. Contrarily, the helical configuration of neighboring metal centers in compound 1 are opposite (Fig. 2): one is Δ-configuration, and the other is Λ-configuration. Undoubtedly, the chiral information could not be transferred along the 1D chain in compound 1.
![ORTEP diagram of compound 2a and illustration of the chiral environment around Dy1. The neighboring {Q[5]Dy(H2O)4Q[5]}3+ cations have same helical chirality. Displacement ellipsoids are drawn at the 30% probability level. H atoms, solvate water molecules and anions are omitted for clarity.](/image/article/2012/RA/c2ra01132h/c2ra01132h-f4.gif) | ||
| Fig. 4 ORTEP diagram of compound 2a and illustration of the chiral environment around Dy1. The neighboring {Q[5]Dy(H2O)4Q[5]}3+ cations have same helical chirality. Displacement ellipsoids are drawn at the 30% probability level. H atoms, solvate water molecules and anions are omitted for clarity. | ||
![X-ray structures of the Q[5]-Dy(iii) complexes in the compound 2a. The five carbonyl-oxygen planes of the portals, and hydrogens are omitted for clarity.](/image/article/2012/RA/c2ra01132h/c2ra01132h-f5.gif) | ||
| Fig. 5 X-ray structures of the Q[5]-Dy(III) complexes in the compound 2a. The five carbonyl-oxygen planes of the portals, and hydrogens are omitted for clarity. | ||
We also investigated the interactions of Hyq molecules with the other components in the compound 2a. Fig. 6a shows the side view of a Q[5]-based helical polymer, in which the Hyq molecules circle around the helical polymer and form a Hyq-based right-handed helix. Moreover, the Hyq-helix seems to be fixed inside the channel of the Q[5]-based helical polymer (Fig. 6b). The most interesting feature of compound 2a is that each Q[5]-based helical polymer is surrounded by six neighbors, as shown in Fig. 6c and 6d. A closer inspection reveals that such a stack arrangement could be stabilized by the π⋯π stacking interactions between the phenyl ring of Hyq and the carbonyl group of Q[5], and the C–H⋯π interactions between the phenyl ring of Hyq and the methine groups of Q[5].
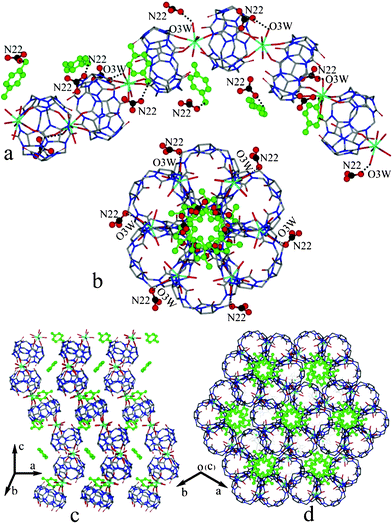 | ||
| Fig. 6 X-ray structures of the chiral helical coordination polymer with Hyq and counter-anions: (a) side view and (b) top view; stacking of the helical polymers: (c) side view and (d) top view of the compound 2a. Hydrogens are omitted for clarity. | ||
It should be noted that the Q[5]-based helical polymers in the compound 2a are all right-hand helices. To the best of our knowledge, this is the first example of a Q[n]-based chiral helical coordination polymer constructed from a symmetric organic molecule. On the basis of the above observations, it can be concluded that the formation of the chiral helical structure of compound 2 may be attributed to the inducement of the Hyq molecule. The high affinity of the Hyq molecule to the Q[5] and steric effect of Hyq molecule induced an appropriate coordination mode of Dy3+ ion. In other words, the Dy3+ ion acts as a chirogenic center, which is the essential reason for the formation of the chiral helix.
It is well known that solid-state circular dichroism (CD) spectroscopy is the most effective method to calculate enantiomeric excess values. However, investigation using solid-state CD spectroscopy in a KBr pellet showed that every single crystal of compound 2 was CD-silent (S3 in ESI). Therefore, one possible but very time-consuming method, single crystal X-ray diffraction analysis, was used to determine the absolute configuration of all single crystals in one batch. Our results revealed that every single crystal in a single cluster of crystals is of the same space group and displayed the same absolute structure, suggesting chiral symmetry breaking. To a random beaker of compound 2, crystallographic analysis revealed that only two clusters crystallized in the space group P65 whereas the other six clusters crystallized in the space group P61 (S4 in ESI). In other words, the sample in the present case had undergone spontaneous resolution with an enantiomeric excess value of 50%. According to the theory of coin flipping,11c we can obtain a cluster of homochiral single crystals (S5 in ESI) through careful control of the crystal growth rate. So, the enantiomeric excess value of compound 2 is stochastic and variable.
In summary, we have successfully obtained a pair of homochiral 1D-helical coordination polymers of cucurbit[5]uril with Dy3+ by simply introducing the achiral organic molecule Hyq into the reaction system. The comparative experiments reveal that the achiral organic molecule Hyq acts as an inducer of chiral helix. The method to spontaneous chiral resolution reported here may well open the way to construct enantiomerically pure materials. For example, the synthetic strategy employed here appears very likely to be applicable to the use of homologues and derivatives of Q[5] together with metal ions other than Dy3+, and also with organic helical inducers other than Hyq. We are at present embarking on studies of this type.
This work was supported by the National Natural Science Foundation of China (Grant No. 20662003, 20961002 and 20971002), the Science and Technology Fund of Guizhou Province, Natural Science Foundation of the department of Education of Guizhou Province, the International Collaborative Project of Guizhou Province (No. 2007400108) and the “Chun-Hui” Funds of Chinese Ministry of Education.
References
- (a) R. Noyori, Asymmetric Catalysis in Organic Synthesis; John Wiley & Sons: New York, 1994 Search PubMed; (b) J. M. Lehn, Supramolecular Chemistry; VCH: Weinheim, Germany, 1995 Search PubMed; (c) D. B. Cline, Physical Origin of Homochirality in Life, American Institute of Physics, New York, 1996 Search PubMed.
- (a) B. Kesanli and W. Lin, Coord. Chem. Rev., 2003, 246, 305 CrossRef CAS; (b) M. E. Davis, Nature, 2002, 417, 813 CrossRef CAS; (c) G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS; (d) L. J. Prins, J. Huskens, F. Jong, P. Timmerman and D. N. Reinhoudt, Nature, 1999, 398, 498 CrossRef CAS; (e) H. Engelkamp, S. Middelbeek and R. J. M. Nolte, Science, 1999, 284, 785 CrossRef CAS.
- (a) J. M. Ribó, J. Crusats, F. Sagués, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef; (b) J. Chin, S. S. Lee, K. J. Lee, S. Park and D. H. Kim, Nature, 1999, 401, 254 CrossRef CAS.
- (a) C. B. France and B. A. Parkinson, J. Am. Chem. Soc., 2003, 125, 12712 CrossRef CAS; (b) T. Nakanishi, N. Yamakawa, T. Asahi, T. Osaka, B. Ohtani and K. Uosaki, J. Am. Chem. Soc., 2002, 124, 740 CrossRef CAS.
- (a) I. Weissbuch, L. Leiserowitz and M. Lahav, Top. Curr. Chem., 2005, 259, 123 CrossRef CAS; (b) R. Noyori, Angew. Chem., Int. Ed., 2002, 41, 2008 CrossRef CAS; (c) W. S. Knowles, Angew. Chem., Int. Ed., 2002, 41, 1998 CrossRef CAS; (d) K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2024 CrossRef CAS.
- http://en.wikipedia.org/wiki/Thalidomide .
- (a) For example: A. L. Nuzhdin, D. N. Dybtsev, K. P. Bryliakov, E. P. Talsi and V. P. Fedin, J. Am. Chem. Soc., 2007, 129, 12958 CrossRef CAS; (b) X. K. Fang, T. M. Anderson and C. L. Hill, Angew. Chem., Int. Ed., 2005, 44, 3540 CrossRef CAS; (c) A. G. Hu, G. T. Yee and W. B. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS; (d) C. D. Wu and W. B. Lin, Angew. Chem., Int. Ed., 2005, 44, 1958 CrossRef CAS.
- (a) K. Tanaka, S. Oda and M. Shiro, Chem. Commun., 2008, 820 RSC; (b) C. D. Wu and W. B. Lin, Angew. Chem., Int. Ed., 2007, 46, 1075 CrossRef CAS; (c) W. B. Lin, MRS Bull., 2007, 32, 544 CrossRef CAS; (d) D. N. Dybtsev, A. L. Nuzhdin, H. Chun, K. P. Bryliakov, E. P. Talsi, V. P. Fedin and K. Kim, Angew. Chem., Int. Ed., 2006, 45, 930 CrossRef; (e) C. D. Wu, A. G. Hu, L. Zhang and W. B. Lin, J. Am. Chem. Soc., 2005, 127, 8940 CrossRef CAS; (f) H. K. Ngo and W. B. Lin, Top. Catal., 2005, 34, 85 CrossRef CAS; (g) W. B. Lin, J. Solid State Chem., 2005, 178, 2486 CrossRef CAS.
- For example: (a) Y. Liu, X. Xu, F. Zheng and Y. Cui, Angew. Chem., Int. Ed., 2008, 47, 4538 CrossRef CAS; (b) S. Galli, N. Masciocchi, E. Cariati, A. Sironi, E. Barea, M. A. Haj, J. A. R. Navarro and J. M. Salas, Chem. Mater., 2005, 17, 4815 CrossRef CAS; (c) Q. Ye, Y. H. Li, Q. Wu, Y. M. Song, J. X. Wang, H. Zhao, R. G. Xiong and Z. L. Xue, Chem.–Eur. J., 2005, 11, 988 CrossRef CAS; (d) B. J. Coe and N. R. M. Curati, Comments Inorg. Chem., 2004, 25, 147 CrossRef CAS; (e) T. C. Shehee, R. E. Sykora, M. K. Kang, P. S. Halasyamani and T. E. Albrecht-Schmitt, Inorg. Chem., 2003, 42, 457 CrossRef CAS; (f) O. R. Evans and W. B. Lin, Acc. Chem. Res., 2002, 35, 511 CrossRef CAS.
- (a) For example: X. L. Tong, T. L. Hu, J. P. Zhao, Y. K. Wang, H. Zhang and X. H. Bu, Chem. Commun., 2010, 46, 8543 RSC; (b) C. Train, R. Gheorghe, V. Krstic, L. M. Chamoreau, N. S. Ovanesyan, G. L. J. A. Rikken, M. Gruselle and M. Verdaguer, Nat. Mater., 2008, 7, 729 CrossRef CAS; (c) E. Q. Gao, Y. F. Yue, S. Q. Bai, Z. He and C. H. Yan, J. Am. Chem. Soc., 2004, 126, 1419 CrossRef CAS; (d) E. Q. Gao, S. Q. Bai, Z. M. Wang and C. H. Yan, J. Am. Chem. Soc., 2003, 125, 4984 CrossRef CAS; (e) E. Coronado, F. Palacio and J. Veciana, Angew. Chem., Int. Ed., 2003, 42, 2570 CrossRef CAS; (f) L. D. Barron, Nature, 2000, 405, 895 CrossRef CAS; (g) G. L. J. A. Rikken and E. Raupach, Nature, 2000, 405, 932 CrossRef CAS.
- (a) For example: W.-T. Liu, Y.-C. Ou, Z.-J. Lin and M.-L. Tong, CrystEngComm, 2010, 12, 3487 RSC; (b) J. Zhang, S. M. Chen, T. Wu, P. Y. Feng and X. H. Bu, J. Am. Chem. Soc., 2008, 130, 12882 CrossRef CAS; (c) S. MacQuarrie, M. P. Thompson, A. Blanc, N. J. Mosey, R. P. Lemieux and C. M. Crudden, J. Am. Chem. Soc., 2008, 130, 14099 CrossRef CAS; (d) X.-Z. Li, M. Li, Z. Li, J. Z. Hou, X. C. Huang and D. Li, Angew. Chem., Int. Ed., 2008, 47, 6371 CrossRef CAS; (e) C. He, Y.-G. Zhao, D. Guo, Z.-H. Lin and C.-Y. Duan, Eur. J. Inorg. Chem., 2007, 3451 CrossRef CAS; (f) Z. J. Lin, A. M. Z. Slawin and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 4880 CrossRef CAS; (g) A. G. Hu, H. L. Ngo and W. B. Lin, J. Am. Chem. Soc., 2003, 125, 11490 CrossRef CAS; (h) A. G. Hu, H. L. Ngo and W. B. Lin, Angew. Chem., Int. Ed., 2003, 42, 6000 CrossRef CAS.
- (a) A. Lennartson and M. Hakansson, Angew. Chem., Int. Ed., 2009, 48, 5869 CrossRef CAS; (b) A. Lennartson and M. Hakansson, CrystEngComm, 2009, 11, 1979 RSC; (c) S. T. Wu, Y. R. Wu, Q. Q. Kang, H. Zhang, L. S. Long, Z. P. Zheng, R. B. Huang and L. S. Zheng, Angew. Chem., Int. Ed., 2007, 46, 8475 CrossRef CAS; (d) A. Lennartson, M. Vestergren and M. Hakansson, Chem.–Eur. J., 2005, 11, 1757 CrossRef CAS; (e) D. K. Kondepudi and K. Asakura, Acc. Chem. Res., 2001, 34, 946 CrossRef CAS; (f) M. Hakansson, M. Vestergren, B. Gustafsson and G. Hilmersson, Angew. Chem., Int. Ed., 1999, 38, 2199 CrossRef CAS; (g) D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975 CAS.
- (a) J. Kim, I. S. Jung, S. Y. Kim, E. Lee, J. K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540 CrossRef CAS; (b) A. I. Day, A. P. Arnold, R. J. Blanch and B. Snushall, J. Org. Chem., 2001, 66, 8094 CrossRef CAS.
- (a) Reviews: W. L. Mock Comprehensive Supramolecular Chemistry, Pergamon, Oxford, 1996, 2, 477 Search PubMed; (b) O. A. Gerasko, D. G. Samsonenko and V. P. Fedin, Russ. Chem. Rev., 2002, 71, 741 CrossRef CAS; (c) K. Kim, Chem. Soc. Rev., 2002, 31, 96 RSC; (d) J. W. Lee, S. Samal, N. Selvapalam, H. J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS; (e) J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS , and references therein.
- (a) D. Whang, J. Heo, C. A. Kim and K. Kim, Chem. Commun., 1997, 2361 RSC; (b) K. M. Park, D. Whang, E. Lee, J. Heo and K. Kim, Chem.–Eur. J., 2002, 8, 498 CrossRef CAS.
- F. Zhang, T. Yajima, Y. Z. Li, G. Z. Xu, H. L. Chen, Q. T. Liu and O. Yamauchi, Angew. Chem., Int. Ed., 2005, 44, 3402 CrossRef CAS.
- X. Xiao, J. X. Liu, Z. F. Fan, K. Chen, Q. J. Zhu, S. F. Xue and Z. Tao, Chem. Commun., 2010, 46, 3741 RSC.
- J. P. Zeng, H. Cong, K. Chen, S. F. Xue, Y. Q. Zhang, Q. J. Zhu, J. X. Liu and Z. Tao, Inorg. Chem., 2011, 50, 6521 CrossRef CAS.
- (a) J. X. Liu, Y. F. Hu, R. L. Lin, W. Q. Sun, X. H. Liu and W. R. Yao, J. Coord. Chem., 2010, 63, 1369 CrossRef CAS; (b) J. X. Liu, Y. F. Gu, R. L. Lin, W. R. Yao, X. H. Liu and J. Zhu, Supramol. Chem., 2010, 22(2), 130 CrossRef CAS; (c) J. X. Liu, L. S. Long, R. B. Huang and L. S. Zheng, Inorg. Chem., 2007, 46, 10168 CrossRef CAS; (d) J. X. Liu, L. S. Long, R. B. Huang and L. S. Zheng, Cryst. Growth Des., 2006, 6, 2611 CrossRef CAS.
- K. Chen, L. L. Liang, Y. Q. Zhang, Q. J. Zhu, S. F. Xue and Z. Tao, Inorg. Chem., 2011, 50, 7754 CrossRef CAS.
- (a) H. D. Flack, Helv. Chim. Acta, 2003, 86, 905 CrossRef CAS; (b) H. D. Flack, Acta Crystallogr., Sect. A: Found. Crystallogr., 1983, 39, 876 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Syntheses of the compounds 1 and 2, X-ray analysis details, single crystal X-ray crystallographic data. CCDC reference numbers 820006 for 1, CCDC 820005 for 2a and CCDC 844326 for 2b. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra01132h |
| This journal is © The Royal Society of Chemistry 2012 |
