Selective thioether macrocyclization of peptides having the N-terminal 2-chloroacetyl group and competing two or three cysteine residues in translation†‡
Abstract
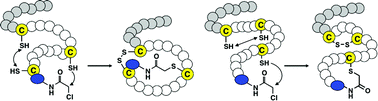
The mode of thioether macrocyclization of
- This article is part of the themed collection: Organic & Biomolecular Chemistry 10th Anniversary

 Please wait while we load your content...
Please wait while we load your content...