One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light†
Jianhua
Shen
,
Yihua
Zhu
*,
Xiaoling
Yang
,
Jie
Zong
,
Jianmei
Zhang
and
Chunzhong
Li
Key Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science and Technology, Shanghai 200237, China. E-mail: yhzhu@ecust.edu.cn; czli@ecust.edu.cn; Fax: +86 21 6425 0624; Tel: +86 21 6425 2022
First published on 25th October 2011
Abstract
A novel and simple approach for preparing graphene quantum dots surface-passivated by polyethylene glycol (GQDs-PEG) has been developed by a one-pot hydrothermal reaction, using small graphene oxide (GO) sheets and polyethylene glycol (PEG) as starting materials. The prepared GQDs-PEG show excellent luminescence properties, the PL quantum yield of the GQDs-PEG with 360 nm emission was about 28.0%, which was two times higher than the pure GQDs. Interestingly, the GQDs-PEG possess the upconversion photoluminescence (UCPL) properties. Blue PL was clearly shown both under the ultraviolet and 808 nm laser in the fluorescent microscopy images. So the GQDs-PEG may provide a new type of fluorescence and upconversion material for applications in bioscience and energy technology. Especially, the GQDs-PEG showed higher photocurrent generation capability. And their photoelectrode generated an obvious and stable photocurrent under a 808 nm near-infrared (NIR) laser. Due to the low cost and simple method, GQDs-PEG thus provide a cost-effective dopant material for solar energy conversion.
1. Introduction
Graphene, a single layer of carbon atoms in a honeycomb structure, has generated enormous excitement due to its large surface area, high carrier transport mobility, superior mechanical flexibility and excellent thermal/chemical stability.1 The size-dependent bandgap of graphenes was particularly interesting for photovoltaic applications. With bulk graphene having a zero bandgap, in principle the bandgap of graphenes can be tuned from 0 eV to that of benzene by varying their sizes.2,3 Interestingly, they also possess upconversion photoluminescence (PL) properties. Graphenes with these unique properties could be an efficient platform for the photoelectrochemical (PEC) cells.To facilitate the application of graphene in nanodevices and effectively tune the bandgap of graphenes, a promising approach is to convert the 2D graphene sheets into 0D graphene quantum dots (GQDs). However, these GQDs exhibited a new phenomenon due to the quantum confinement and edge effects. And GQDs are similar to carbon quantum dots (CQDs). Over the past few decades, CQDs are attracting considerable attention as nascent quantum dots, as they may gradually replace traditional semiconductor quantum dots due to their superiority in chemical inertness, biocompatibility and low toxicity.4–8 However, the disadvantage is that CQDs possess size effects, and quasispherical nanoparticles with sizes below 10 nm.4 Therefore, the GQDs and their chemical derivatives have received more attention, as their diameters are mainly distributed in a larger range (3–20 nm).9–16 In addition, Dai and co-workers9 described intrinsic luminescence from single layer nanographene oxide (NGO) sheets surface-passivated by polyethylene glycol (PEG) in both the visible and NIR regions. Kikkawa and colleagues10 reported a hydrazine vapor reduction of graphene oxide (GO) into the GQDs with broadband visible PL possessing a long NIR emission. Gokus et al.11 discovered the induction of pronounced PL within single-layer graphene flakes treated with an oxygen/argon plasma. Qu et al.12 reported an electrochemical approach for direct preparation of functional GQDs with a uniform size of 3–5 nm. Li et al.13,14 demonstrated a solubilization strategy for GQDs, which proved to be attractive for efficient light harvesting in photovoltaics. But these complex processes are difficult for mass production. Pan et al.15 developed a simple hydrothermal route for cutting preoxidized micrometre-sized rippled graphene sheets into ultrafine graphene quantum dots (GQDs). However, the produced GQDs emit strongly under alkaline conditions whereas they are almost completely quenched in acidic media. Very recently, our group reported a method to prepare GQDs surface-passivated by polyethylene glycol (GQDs-PEG) by hydrazine hydrate reduction of GO.16
Here we present a simple and novel method to prepare GQDs-PEG by a one-pot hydrothermal reaction, using small GO sheets and PEG as starting materials. GO sheets were reduced to GQDs and the hydroxyl groups (−OH) of PEG were connected to the carboxyl groups (−COOH) of GQDs simultaneously.9,15,17 For comparison, we also prepared GQDs without the surface-passivation agent (similar to that reported by Pan et al.; Fig. S1a in ESI†).15 Strong blue PL was clearly shown both in the GQDs and GQDs-PEG under 365 nm. And the PL quantum yield of the GQDs-PEG with 360 nm emission was about 28.0%, which was two times higher than the GQDs (ca. 13.1%), comparable with those of reported luminescent nanoparticles.18–21 Interestingly, the upconverted PL properties of GQDs-PEG were much better than those of GQDs, which were similar to the report.16 In addition, Fig. S1b (ESI†) shows the pictures of the final large aggregate products under sunlight and 365 nm UV lamp. Such a novel and simple hybrid material with strong luminescence would find significant applications in PEC cells. So we also demonstrated that GQDs-PEG had higher photon-to-electron conversion capability compared with GQDs. Interestingly, the GQDs-PEG photoelectrode generated an obvious and stable photocurrent under a 808 nm near-infrared (NIR) laser.
2. Experimental
2.1. Reagents and materials
Graphite powder and PEG 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were purchased from Sinopharm Chemical Reagent Co., Ltd. And all other chemicals were purchased from Shanghai Chemical Reagent Co. All chemicals were used as received without any further purification. Ultrapure water (18 MΩ cm−1) was used for all experiments.
000 were purchased from Sinopharm Chemical Reagent Co., Ltd. And all other chemicals were purchased from Shanghai Chemical Reagent Co. All chemicals were used as received without any further purification. Ultrapure water (18 MΩ cm−1) was used for all experiments.
2.2. Preparation of GQDs surface-passivated by PEG
GO was prepared from natural graphite powder by a modified Hummers method.22,23 The sample GO (0.05 g) was treated in an aqueous nitric acid solution (up to 2.6 M) (40 mL) with 70 °C reflux for 24 h. After cooling to room temperature, the suspension was subjected to mild ultrasonication. The pH was tuned to 7 with NaOH. The suspension was disrupted into small pieces using an ultrasonic cell crusher for 60 min. The sample was filtered through a 0.22 μm microporous membrane to remove the large tracts of GO.In a typical reaction, PEG 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (0.2 g) was mixed with the cutting GO solution (50 mL), and the mixture was substantially mixed. The mixture solution was then transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 200 °C for 24 h. After cooling to room temperature, the resulting solution was further dialyzed in a dialysis bag (retained molecular weight: 14
000 (0.2 g) was mixed with the cutting GO solution (50 mL), and the mixture was substantially mixed. The mixture solution was then transferred into a 100 mL Teflon-lined stainless-steel autoclave and heated at 200 °C for 24 h. After cooling to room temperature, the resulting solution was further dialyzed in a dialysis bag (retained molecular weight: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) for a week and the GQDs-PEG showed strong blue fluorescence. We also prepared the GQDs without surface-passivation for the comparison.
000 Da) for a week and the GQDs-PEG showed strong blue fluorescence. We also prepared the GQDs without surface-passivation for the comparison.
2.3. Photocurrent response measurements
Commercial indium tin oxide (ITO) was used as the substrate for electrode buildup, which was cleaned by sonication sequentially for 20 min each in acetone, 10% KOH in ethanol and doubly deionized water. After the treatment, the solution of GQDs-PEG and GQDs was spin-coated on the ITO. PEC cells were measured in a three-electrode experimental system, consisting of a QDs/ITO working electrode, a platinum wire auxiliary electrode, and an Ag/AgCl (3 M KCl) reference electrode. The electrolyte used in PEC cells was 0.1 M phosphate buffer solution (PBS) at pH = 6.8. A 365 nm UV lamp (70 μW cm−2) was used as the light source. The photocurrent signal was recorded with a CHI 660C workstation (CH Instruments, Chenhua, Shanghai, China) connected to a personal computer. All electrochemical experiments were carried out at room temperature.2.4. Characterizations
High-resolution TEM images were taken on a JEOL JEM 2011 microscope (JEOL, Japan) at an acceleration voltage of 200 kV. The specimen was prepared by drop casting the sample dispersion onto a carbon-coated copper grid, followed by drying under room temperature. PL spectra were measured on a Fluorolog-3-P UV-VIS-NIR fluorescence spectrophotometer (Jobin Yvon, France). The FT-IR spectrum was recorded on a Nicolet 5700 Fourier transform infrared spectrometer and the XRD spectrum of GQDs-PEG was investigated by X-ray powder diffraction (RIGAK, D/MAX 2550 VB/PC, Japan). The UV-vis spectrum of the GQDs dispersed in water was obtained using a Unico UV-2102PC spectrophotometer. Fluorescent microscopy images of GQDs-PEG were recorded on a fluorescence inverse microscope (Nikon TE-2000 U). All spectra were recorded with quartz cells of 10 mm path length.3. Results and discussion
GQDs-PEG were prepared by a one-pot hydrothermal reduction of GO sheets and PEG (Mw = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). And then the precursor removed excess PEG by a 14
000). And then the precursor removed excess PEG by a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-molecular-weight cutoff dialysis bag overnight. Fig. 1a shows the TEM image of the as-prepared GQDs-PEG. The collected GQDs-PEG are nearly monodispersed with a uniform diameter of ca. 5–25 nm (13.0 nm average diameter, similar to the reported).15,16 The absence of discernible lattice structures on the enlarged HRTEM image (Fig. 1b) indicates that the resultant GQDs-PEG are amorphous, which is further confirmed by the diffuse ring pattern obtained by selected area electron diffraction (SAED; inset in Fig. 1a). Fig. 1d–e show the fluorescent microscope images of GQDs-PEG with the ultraviolet (UV) and 808 nm NIR excitation lights from left to right, respectively. Blue PL is clearly shown under the ultraviolet and the dark blue fluorescence is observed under a 808 nm NIR laser, which is consistent with the PL spectra. The bright dots are the GQDs-PEG aggregates, and the larger aggregates are also shown in the Fig. S1 in ESI.† The X-ray diffraction pattern shows that the (002) interlayer spacing is 3.81 Å (Fig. 2a) between the reduced graphene sheets (3.68 Å) and the oxidized graphene sheets (3.97 Å). The (002) interlayer spacing of GQDs-PEG is larger than the reduced graphene sheets. Before the hydrothermal treatment, the oxidized graphene sheets were oxidized in concentrated HNO3, and the (002) spacing increased to 3.97 Å. After the hydrothermal treatment and the surface-passivation by PEG, the polymer chains were introduced at the edge and on the basal plane. As shown in the Fourier transform infrared (FTIR) spectrum (Fig. 2b), the peaks of PEG were shown in the GQDs-PEG, and the peak at 1637 cm−1 is attributed to the benzene ring vibration of C
000-molecular-weight cutoff dialysis bag overnight. Fig. 1a shows the TEM image of the as-prepared GQDs-PEG. The collected GQDs-PEG are nearly monodispersed with a uniform diameter of ca. 5–25 nm (13.0 nm average diameter, similar to the reported).15,16 The absence of discernible lattice structures on the enlarged HRTEM image (Fig. 1b) indicates that the resultant GQDs-PEG are amorphous, which is further confirmed by the diffuse ring pattern obtained by selected area electron diffraction (SAED; inset in Fig. 1a). Fig. 1d–e show the fluorescent microscope images of GQDs-PEG with the ultraviolet (UV) and 808 nm NIR excitation lights from left to right, respectively. Blue PL is clearly shown under the ultraviolet and the dark blue fluorescence is observed under a 808 nm NIR laser, which is consistent with the PL spectra. The bright dots are the GQDs-PEG aggregates, and the larger aggregates are also shown in the Fig. S1 in ESI.† The X-ray diffraction pattern shows that the (002) interlayer spacing is 3.81 Å (Fig. 2a) between the reduced graphene sheets (3.68 Å) and the oxidized graphene sheets (3.97 Å). The (002) interlayer spacing of GQDs-PEG is larger than the reduced graphene sheets. Before the hydrothermal treatment, the oxidized graphene sheets were oxidized in concentrated HNO3, and the (002) spacing increased to 3.97 Å. After the hydrothermal treatment and the surface-passivation by PEG, the polymer chains were introduced at the edge and on the basal plane. As shown in the Fourier transform infrared (FTIR) spectrum (Fig. 2b), the peaks of PEG were shown in the GQDs-PEG, and the peak at 1637 cm−1 is attributed to the benzene ring vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The broad peak centered at 2888 cm−1 is assigned to the stretching vibrations of C–H bonds, while the peak at 3434 cm−1 is attributed to the bending vibrations of O–H bonds. In addition, the peak of O–H (3434 cm−1) in the GQDs-PEG is higher than in the PEG, and the hydrothermally treated GQDs are not completely reduced and a lot of −OH remained. The presence of these groups and PEG makes the GQDs-PEG well soluble in water.
C bonds. The broad peak centered at 2888 cm−1 is assigned to the stretching vibrations of C–H bonds, while the peak at 3434 cm−1 is attributed to the bending vibrations of O–H bonds. In addition, the peak of O–H (3434 cm−1) in the GQDs-PEG is higher than in the PEG, and the hydrothermally treated GQDs are not completely reduced and a lot of −OH remained. The presence of these groups and PEG makes the GQDs-PEG well soluble in water.
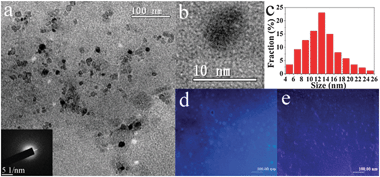 | ||
| Fig. 1 (a) TEM image of the GQDs-PEG, the inset is the corresponding selected area electron diffraction (SAED) pattern; (b) HRTEM image of an individual GQD–PEG; (c) diameter distribution of the GQDs-PEG; (d, e) fluorescent microscopy images of GQDs-PEG under the UV (330–400 nm) light and 808 nm NIR laser (scale bar: 100 μm). | ||
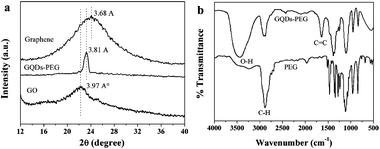 | ||
| Fig. 2 (a) The XRD spectra of the grapheme, GQDs-PEG and GO. (b) The FT-IR spectra of the GQDs-PEG and PEG. | ||
To further explore the optical properties of as-synthesized GQDs-PEG, a detailed PL study was carried out using different excitation wavelengths. Fig. 3a shows the normal and normalized PL spectra of GQDs-PEG. The as-prepared GQDs-PEG also exhibit an excitation-dependent PL behavior. When the excitation wavelength was changed from 300 to 400 nm, the PL peaks shifted from 377 (black) to 493 nm (navy). GQDs show the same phenomenon, but the PL peaks are narrower and their intensity decreases rapidly, and the strongest excitation wavelength has a blue shift from 340 nm to 320 nm (Fig. 3b). Another marked difference involves the UV–vis absorption of the GQDs-PEG and the GQDs dispersed in water (Fig. 3c). For the GQDs, an absorption band at ca. 320 nm is clearly observed, which is similar to that reported by Pan et al.15 But the GQDs-PEG have no clear absorption peak but a long absorption edge. And the threshold wavelength of GQDs-PEG notably shifts from 343 to 323 nm when GQDs were surface-passivated by PEG. The blue shift in threshold wavelength indicates that the surface-passivation can increase the energy gap of GQDs. The PL intensity of the GQDs-PEG is higher than the GQDs under the 365 nm excitation, and the photographs also show the GQD-PEG aqueous solution has a brighter blue fluorescence under 365 nm UV light. By selecting rhodamine B in water as the standard and 360 nm as the excitation wavelength, the PL quantum yield of the GQDs-PEG was measured and calculated to be 28.0% (Table S1 in ESI†), which was beyond two times higher than the GQDs (ca. 13.1%). The value is higher than previously reported for GQDs.15 Due to the surface-passivation agent, there must be a more quantum confinement of emissive energy trapped to the GQDs surface, namely, the GQDs with the surface-passivation exhibit stronger PL.18 Both the GQDs-PEG and GQDs were shown to possess the upconversion PL properties at the same concentration of the solution. Fig. 3d shows the PL spectrum of GQDs-PEG excited by 808 nm with the upconverted emissions located at about 464 nm, and its intensity over 10 times higher than the GQDs.
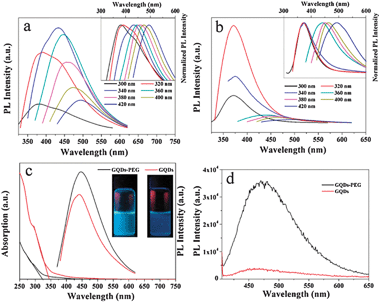 | ||
| Fig. 3 (a, b) PL emission spectra for the GQDs-PEG and GQDs at different excitation wavelengths. Inset: the corresponding normalized PL spectra. (c) UV–vis absorption and PL (at 360 nm excitation) spectra of the GQDs-PEG and GQDs dispersed in water. Inset: photographs of the GQDs-PEG and GQDs aqueous solutions taken under visible light. (d) Upconverted PL spectrum of the GQDs-PEG and GQDs excited at 808 nm. | ||
Remarkably, the GQDs-PEG are shown to possess stronger UCPL properties, and we conducted further studies on the upconversion performance. As shown in Fig. 4a, when the excitation wavelength changed from 700 to 980 nm, the upconverted emission peaks shifted from 431 to 544 nm, respectively. And the shifting between the energy of upconverted emission light (Em) and excitation light (Ex) was almost unchanged, about 1.1 eV. Fig. 4b shows the linear relationship between Em and Ex, and the function of the fit line is Em = 1.00Ex + δE (R2 = 0.9747) with δE = 1.1 eV. This result is consistent with our previous report.16 We speculate on the upconversion luminescence due to the anti-Stokes PL, where the δE between the π and σ orbitals is near 1.1 eV.16,24–27 These are very novel phenomena of optical properties in GQDs-PEG, which are different from the report that the upconverted PL property of CQDs should be attributed to the multiphoton active process.6,28
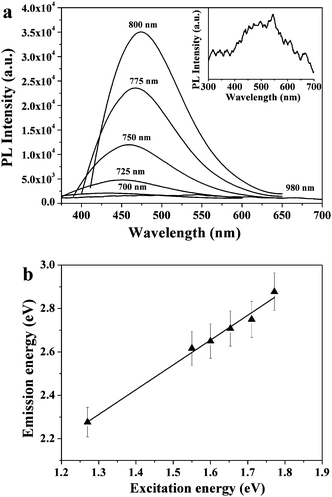 | ||
| Fig. 4 (a) Upconverted PL properties of the GQDs-PEG, inset is the enlarged image of the PL spectrum excited at a 980 nm laser. (b) The energy of the excitation light as a function of the emission, and the function of the fit line is Em = 1.00Ex + δE (R2 = 0.9747) with δE = 1.1 eV. | ||
Since the GQDs-PEG possessed strong luminescence and peculiar upconversion PL, they would find significant applications in PEC cells.29,30 So we also compared the photon-to-electron conversion capability of the GQDs-PEG and GQDs. The GQDs-PEG and GQDs were spin-coated on an ITO substrate. Upon exposure to light, electron–hole pairs were generated and disassociated in the GQDs-PEG, and then the electrons were transported to ITO electrodes. Photocurrents generated by GQDs-PEG and GQDs on ITO electrodes were measured by the three-electrode system. As demonstrated in Fig. 5, GQDs-PEG photoelectrode generated photocurrent with higher density at 365 nm while the pure GQD photoelectrode showed less than half of the photocurrent of the GQDs-PEG. This result is consistent with their PL quantum yield. Meanwhile, we also demonstrated the photoelectric conversion of the GQDs-PEG under the 808 nm NIR laser. In contrast to the ultraviolet, it shows a relatively small and stable photocurrent. This means that GQDs-PEG will be a new solar cell dopant material, the light of the photon-to-electron conversion may be extended from the ultraviolet to the near infrared.
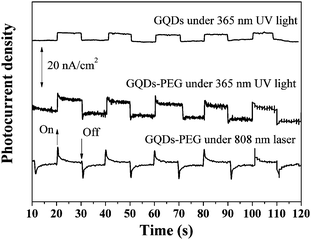 | ||
| Fig. 5 Photocurrent response of the GQDs-PEG and GQDs photoelectrodes under 365 nm UV light or 808 nm NIR laser. | ||
4. Conclusions
In summary, GQDs-PEG were prepared by a one-pot hydrothermal reaction and applied to construct the photoelectrode. The GQDs-PEG photoelectrode generated a relatively small photocurrent under a 808 nm NIR laser, and had higher photon-to-electron conversion capability compared with the pure GQDs. Compared with the GQDs, the GQDs-PEG possessed higher fluorescence performance and upconversion properties. GQDs-PEG may provide a new type of fluorescence and upconversion material for applications in bioscience and energy technology. Especially, the GQDs-PEG showed higher photocurrent generation capability. Due to the low cost and simple method, GQDs-PEG thus provide a cost-effective dopant material for solar energy conversion and other PEC cells applications.This work was supported by the National Natural Science Foundation of China (20925621, 20976054 and 21176083), the Innovation Program of Shanghai Municipal Education Commission (09ZZ58), the Fundamental Research Funds for the Central Universities, the Program for Changjiang Scholars and Innovative Research Team in University (IRT0825), and the Shanghai Leading Academic Discipline Project (project number: B502) for financial supports.
Notes and references
- (a) A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS; (b) S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. B. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282 CrossRef CAS; (c) X. J. Xie, H. Bai, G. Q. Shi and L. T. Qu, J. Mater. Chem., 2011, 21, 2057 RSC.
- K. Nakada, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Phys. Rev. B: Condens. Matter, 1996, 54, 17954 CrossRef CAS.
- Y. W. Son, M. L. Cohen and S. G. Louie, Phys. Rev. Lett., 2006, 97, 216803 CrossRef.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726 CrossRef CAS.
- (a) K. Welsher, Z. Liu, D. Daranciang and H. Dai, Nano Lett., 2008, 8, 586 CrossRef CAS; (b) R. Liu, D. Wu, S. Liu, K. Koynov, W. Knoll and Q. Li, Angew. Chem., 2009, 121, 4668 ( Angew. Chem., Int. Ed. , 2009 , 48 , 4598 ) CrossRef; (c) D. R. Larson, W. R. Zipfel, R. M. Williams, S. W. Clark, M. P. Bruchez, F. W. Wise and W. W. Webb, Science, 2003, 300, 1434 CrossRef CAS; (d) H. Peng and J. Travas-Sejdic, Chem. Mater., 2009, 21, 5563 CrossRef CAS; (e) S. C. Ray, A. Saha, N. R. Jana and R. Sarkar, J. Phys. Chem. C, 2009, 113, 18546 CrossRef CAS.
- L. Cao, X. Wang, M. J. Meziani, F. Lu, H. Wang, P. G. Luo, Y. Lin, B. A. Harruff, L. M. Veca, D. Murray, S.-Y. Xie and Y. P. Sun, J. Am. Chem. Soc., 2007, 129, 11318 CrossRef CAS.
- (a) H. Zhu, X. Wang, Y. Li, Z. Wang, F. Yang and X. Yang, Chem. Commun., 2009, 5118 RSC; (b) S. L. Hu, K. Y. Niu, J. Sun, J. Yang, N. Q. Zhao and X. W. Du, J. Mater. Chem., 2009, 19, 484 RSC; (c) Q. L. Zhao, Z. L. Zhang, B. H. Huang, J. Peng, M. Zhang and D. W. Pang, Chem. Commun., 2008, 5116 RSC; (d) S. T. Yang, X. Wang, H. F. Wang, F. S. Lu, P. J. G. Luo, L. Cao, M. J. Meziani, J. H. Liu, Y. Liu, M. Chen, Y. Huang and Y. P. Sun, J. Phys. Chem. C, 2009, 113, 18110 CrossRef CAS; (e) F. Wang, S. P. Pang, L. Wang, Q. Li, M. Kreiter and C. Y. Liu, Chem. Mater., 2010, 22, 4528 CrossRef CAS.
- J. Zong, Y. H. Zhu, X. L. Yang, J. H. Shen and C. Z. Li, Chem. Commun., 2011, 47, 764 RSC.
- X. Sun, Z. Liu, K. Welsher, J. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203 CrossRef CAS.
- Z. T. Luo, P. M. Vora, E. J. Mele, A. T. C. Johnson and J. M. Kikkawa, Appl. Phys. Lett., 2009, 94, 111909 CrossRef.
- T. Gokus, R. R. Nair, A. Bonetti, M. Bohmler, A. Lombardo, K. S. Novoselov, A. K. Geim, A. C. Ferrari and A. Hartschuh, ACS Nano, 2009, 3, 3963 CrossRef CAS.
- Y. Li, Y. Hu, Y. Zhao, G. Q. Shi, L. Deng, Y. B. Hou and L. T. Qu, Adv. Mater., 2011, 23, 776 CrossRef CAS.
- X. Yan, X. Cui and L. S. Li, J. Am. Chem. Soc., 2010, 132, 5944 CrossRef CAS.
- X. Yan, X. Cui, B. S. Li and L. S. Li, Nano Lett., 2010, 10, 1869 CrossRef CAS.
- D. Pan, J. C. Zhang, Z. Li and M. H. Wu, Adv. Mater., 2010, 22, 734 CrossRef CAS.
- J. H. Shen, Y. H. Zhu, C. Chen, X. L. Yang and C. Z. Li, Chem. Commun., 2011, 47, 2580 RSC.
- K. F. Zhou, Y. H. Zhu, X. L. Yang and C. Z. Li, New J. Chem., 2010, 34, 2950 RSC.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. S. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. F. Wang, P. J. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756 CrossRef CAS.
- H. T. Li, X. D. He, Z. H. Kang, H. Huang, Y. Liu, J. L. Liu, S. Y. Lian, C. H. A. Tsang, X. Bao and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430 CrossRef CAS.
- L. Li, H. F. Qian and J. C. Ren, Chem. Commun., 2005, 528 RSC.
- A. P. Alivisatos, Science, 1996, 271, 933 CrossRef CAS.
- Y. Xu, H. Bai, G. Lu, C. Li and G. Shi, J. Am. Chem. Soc., 2008, 130, 5856 CrossRef CAS.
- D. Pan, S. Wang, B. Zhao, M. Wu, H. Zhang, Y. Wang and Z. Jiao, Chem. Mater., 2009, 21, 3137.
- J. R. Santos, M. I. Vasilevskiy and S. A. Filonovich, Phys. Rev. B: Condens. Matter, 2008, 78, 245422 CrossRef.
- D. Bourissou, O. Guerret, F. P. Gabba and G. Bertrand, Chem. Rev., 2000, 100, 39 CrossRef.
- R. Hoffmann, J. Am. Chem. Soc., 1968, 90, 1475 CrossRef CAS.
- X. Y. Wang, W. W. Yu, J. Y. Zhang, J. Aldana, X. G. Peng and M. Xiao, Phys. Rev. B: Condens. Matter, 2003, 68, 125318 CrossRef.
- H. T. Li, X. D. He, Z. H. Kang, H. Huang, Y. Liu, J. L. Liu, S. Y. Lian, C. H. A. Tsang, X. Bao and S. T. Lee, Angew. Chem., Int. Ed., 2010, 49, 4430 CrossRef CAS.
- H. X. Chang, X. J. Lv, H. Zhang and J. H. Li, Electrochem. Commun., 2010, 12, 483 CrossRef CAS.
- H. Nishikiori, Y. Uesugi, S. Takami, R. A. Setiawan, T. Fujii, W. Qian and M. A. El-Sayed, J. Phys. Chem. C, 2011, 115, 2880 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Representation of the samples and quantum yield measurements. See DOI: 10.1039/c1nj20658c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2012 |
