Research highlights
Šeila
Selimović
ab,
Mehmet R.
Dokmeci
ab and
Ali
Khademhosseini
*abcd
aCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, USA. E-mail: alik@rics.bwh.harvard.edu
bHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
cWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, USA
dWorld Premier International-Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai, 980-8577, Japan
First published on 3rd April 2012
Nanovesicles for high throughput handling of zeptoliter volumes
Performing sample preparation, chemical reactions and other laboratory tasks in a miniaturized and high throughput manner is advantageous as it can reduce the amount of costly reagents and increase sample throughput.1,2 Although a range of miniaturization techniques have been developed to reduce the sample size down to the nano-picoliter scale,3 the field could benefit from further development of more miniaturized high throughput systems.4For this purpose, Stamou and colleagues have recently explored the use of self-assembled vesicles as containers for nanoscale samples. In their study, Christensen et al.5 generated small unilamellar lipid vesicles (SUVs) containing different chemical samples and guided them to fuse into larger units and merge their contents. The mixed products were then detected using FRET (Förster resonance energy transfer).
Lipid vesicles (nanoreactors) were formed by extrusion through porous polycarbonate membranes, with the nanoreactor diameters ranging from 15 to 700 nm, thus containing volumes as small as a few zeptoliters ( > 10¬21 l). One set of SUVs was permanently adhered to a glass substrate (acceptor reactors) and another set (donor reactors) containing a different chemical was allowed to diffuse freely. Since the two sets of reactors were formed from oppositely charged lipids, they fused upon contact, which led to mixing of their contents within microseconds. The mixing events were quantified by recording FRET signals from the two sets of SUVs prior to and after fusion. For example, the mixing of an acceptor reactor containing alkaline phosphatase and a donor reactor carrying fluorescein diphosphate led to the generation of a fluorescent reaction product. This product could only be observed after the mixing event and was recorded in 80–90% of individual SUVs in a large sample population.
A similar experimental setup was used to determine whether the chemicals encapsulated inside SUVs leaked. Here, the acceptor reactors contained a water-soluble fluorescent dye. Upon fusion with another reactor, the dye remained confined to the SUV and did not leak through the vesicle membrane. In addition, fusion events could be triggered up to four times using the same acceptor reactors, whereby each time a characteristic fusion period was measured. This observation indicated that sequential chemical reactions could be performed on the same sample in a controlled manner.
The fabrication and loading of the samples is fast and simple, as the vesicles are self-assembled, and the extrusion mechanism offers a relatively narrow size distribution. Although the sample mixing is non-deterministic, this method offers a product detection yield of up to 90%, making it highly precise and reliable. Furthermore, fluorescence signals indicating the presence of reaction products are spatially well resolved and much brighter than the background, which simplifies the optical detection. These characteristics of vesicle-based sample manipulation allow for simultaneous handling of millions of samples in a short time (μs) in a small space (cm2), making this process of great potential value for high-throughput applications. Future applications of this technique could involve sample coding, e.g. using quantum dots, or screening of large libraries of biological and chemical samples.
Microdroplets as sensors
Fluid flow focusing inside microfludic channels can be used to generate droplets that may be beneficial for a range of high-throughput applications.6 Benefits of droplet generation using this approach include high monodispersity of droplets, high droplet generation rates (up to 106 droplets/h),7 the ability to encapsulate small particles and cells, and low sample volumes (pl).8Each droplet can be used as a miniaturized reactor enabling the testing of many different conditions simultaneously. However, a current challenge with this approach is that most observation methods rely on detecting fluorescence signals from the droplets. For example, for many chemical reactions the fluorescent species is either introduced into the sample during the droplet formation or generated within the droplet and the change in the fluorescence intensity is measured over time and is correlated to the rate of the chemical reaction. Alternatively, markers or stains are added at a later time to analyze the droplets, which is cumbersome as it requires washing of the samples or merging of two or more droplets with different contents. Thus, there is a need for label-free methods of analyzing droplets for various types of analysis.
Recently, Böhm and coworkers have used changes in the droplet size as a way of measuring the behavior of encapsulated cells within each droplet. In their experiments, the size of the droplets changed due to reaction-driven changes to solute concentration, which in turn resulted in a difference in osmotic pressure, as previously demonstrated.10 In their study, Hofmann et al.9 generated 10 pl droplets using a two phase microfluidic system, and stored the resulting droplets in a glass incubation chamber that was impermeable to gas and water. The monodisperse droplets contained sucrose solutions with concentrations ranging from 100 to 500 mM. During incubation, the amount of sucrose in each droplet did not change, however, the difference in chemical potential of sucrose in neighboring droplets gave rise to an osmotic pressure difference and permeation of water from droplets with low concentration of sucrose to those with high concentration of the solute. The resulting change in the amount of water in different droplets was indicated by changes in droplet size – high sucrose droplets became larger and low sucrose droplets smaller – which was observable within a few hours. Such a short time scale was possible because of the solubility of water in the surrounding phase, the small droplet size and short separation distance between the droplets.
In another experiment, live and dead yeast cells were encapsulated independently inside media-containing droplets. When live cells were encapsulated inside droplets, the authors observed that the cells proliferated over time. Accordingly, the cells consumed the available nutrients, which resulted in reduction of the droplet size (Fig. 1). As expected, the control studies conducted with empty droplets or droplets encapsulating dead cells did not result in changes of the droplet size.
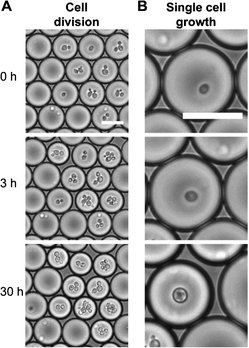 | ||
| Fig. 1 Droplets shrink with time as encapsulated cells consume solutes and lower the solute concentration: cell division (A) and single cell growth (B). Scale bar: 30 μm. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Hofmann et al.9 | ||
The proposed method for label-free detection of reaction byproducts and changes in solutes is simple to implement and only requires a camera for signal detection (changes in drop size). In addition, if the initial solute concentration is known, the real-time concentration of solutes can be measured by comparing the droplet size at that time with its initial size. Thus this platform can be used for tracking chemical reactions as well as cell metabolism and growth. In addition, by using this technique thousands of droplets can be analyzed in a short amount of time by applying simple image-analysis techniques. Therefore, this osmosis-driven analysis method can be utilized both in high-throughput sensing and screening applications, such as protein crystallization. Currently, a limitation of this technique is the potential crosstalk between concentrations of several solutes inside the same drop, such that only the total osmotic pressure can be determined, but not the individual contributions of different molecules. In the future, this shortcoming could be addressed by engineering droplets with semi-permeable membranes that enable more selective permeation of various molecules.
Bioinspired reversibly locking devices
Nature provides the inspiration for numerous novel materials with applications that range from adhesives to mechanical actuators.11,12 Interestingly, by engineering biomimetic and bioinspired systems, our understanding of physical laws and chemical processes in nature is enriched. Furthermore, such systems often enable new properties that were previously not achievable. An example is the ongoing research on interlocking devices based on Velcro-like materials.Recently, Suh and colleagues have engineered reversible interlocking devices by mimicking the wing-locking structures in beetles. Pang et al.13 studied the microscopic surfaces of the beetle thorax and wing and found hexagonal arrays of thin microhairs. They observed that interlocking and subsequent shearing of the microhairs on the wing and the thorax against each other resulted in a large shear force and locking of the two surfaces (Fig. 2). However, this did not affect a lift-off normal to the two surfaces – they could be easily separated by peeling them off each other. To better understand the locking mechanism, the researchers attempted to replicate the microhairs by preparing a range of polyurethane (PU)-based structures with high aspect-ratio pillars. The PU structures were molded from a patterned silicon substrate. The experimental parameters included the pillar diameter (D = 100 nm, 3 μm, and 15 μm), aspect ratio (AR = 3, 6, and 10), and stiffness, as well as the thickness of an additional Pt-coating.
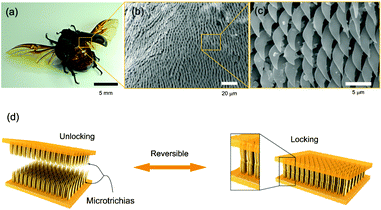 | ||
| Fig. 2 Beetle wing-locking device (a) and SEM photographs of the microtrichia (b, c). d) Schematic of the engineered wing-locking chip. Figure reprinted with permission from Pang et al.13 | ||
By generating a highly dense, hexagonal array of pillars, it was found that the shear adhesion per unit area was comparatively higher for smaller pillar diameters than for large ones. As a consequence, for high density pillars more pillar-to-pillar contacts were made during interlocking, which increased Van-der-Waals interactions and ultimately the adhesion force. The use of softer pillar materials as well as higher aspect ratios also led to a larger shear force. The reversibility of the interlocking mechanism was tested in over 300 locking and release cycles. While no significant reduction in the adhesion force was measured, it was shown that pillars reinforced with a nm-thin Pt coating suffered less structural damage than untreated pillars. More recently, an additional analysis has been presented by the same group regarding preload-dependent interlocking of the wing locking device.14
These results helped to identify the optimal geometry and material properties for a reliable interlocking device that can be operated multiple times. Advantages of this particular structure include the simple geometry, as no microscale hooks or other features are needed to lock the device. Furthermore, since the interaction between interlocking pillars is based chiefly on Van-der-Waals forces, extensive surface treatment is also unnecessary. This makes the developed structure a cost-effective and simple biomimetic device.
References
- R. P. Hertzberg and A. J. Pope, High-throughput screening: new technology for the 21st century., Curr. Opin. Chem. Biol., 2000, 4(4), 445–451 CrossRef CAS.
- B. J. Battersby and M. Trau, Novel miniaturized systems in high-throughput screening., Trends Biotechnol., 2002, 20(4), 167–173 CrossRef CAS.
- D. A. Dunn and I. Feygin, Challenges and solutions to ultra-high-throughput screening assay miniaturization: submicroliter fluid handling., Drug Discovery Today, 2000, 5(12, Supplement 1), 84–91 CrossRef.
- T. Sunami, Femtoliter compartment in liposomes for in vitro selection of proteins., Anal. Biochem., 2006, 357(1), 128–136 CrossRef CAS.
- S. M. Christensen, et al., Mixing subattolitre volumes in a quantitative and highly parallel manner with soft matter nanofluidics., Nat. Nanotechnol., 2012, 7(1), 51–55 CrossRef CAS.
- M. Joanicot and A. Ajdari, Droplet Control for Microfluidics., Science, 2005, 309, 887–888 CrossRef CAS.
- Y. Zeng, et al., High-Performance Single Cell Genetic Analysis Using Microfluidic Emulsion Generator Arrays., Anal. Chem., 2010, 82(8), 3183–3190 CrossRef CAS.
- S.-Y. Park, et al., High-speed droplet generation on demand driven by pulse laser-induced cavitation., Lab Chip, 2011, 11, 1010–1012 RSC.
- T.W. Hofmann, et al., Applying microdroplets as sensors for label-free detection of chemical reactions., Lab Chip, 2012, 12(5), 916–922 RSC.
- J.-u. Shim, et al., Using Microfluidics to Decouple Nucleation and Growth of Protein Crystals., Cryst. Growth Des., 2007, 7(11), 2192–2194 CAS.
- B.-C. Yoseph, Biomimetics—using nature to inspire human innovation., Bioinspir. Biomimetics, 2006, 1(1), P1 CrossRef.
- B. Bhushan, Biomimetics: lessons from nature-an overview., Philos. Trans. R. Soc. London, Ser. A, 1893, 367, 1445–1486 CrossRef.
- C. Pang, et al., Bioinspired Reversible Interlocker Using Regularly Arrayed High Aspect-Ratio Polymer Fibers., Adv. Mater., 2012, 24(4), 475–479 CrossRef CAS.
- C. Pang, et al., Analysis of preload-dependent reversible mechanical interlocking using beetle-inspired wing locking device., Langmuir, 2012, 28(4), 2181–2186 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
