NOx conversion on LSM15-CGO10 cell stacks with BaO impregnation
Abstract
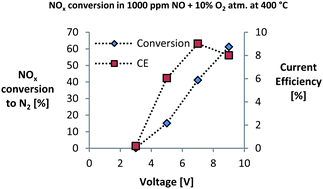
The electrochemical conversion of NOx on non-impregnated and BaO-impregnated LSM15-CGO10 (La0.85Sr0.15MnO3–Ce0.9Gd0.1O1.95) porous cell stacks has been investigated, and extensive impedance analysis have been performed to identify the effect of the BaO on the electrode processes. The investigation was conducted in the temperature range 300–500 °C, a polarisation range from 3 V to 9 V and in atmospheres containing 1000 ppm NO, 1000 ppm NO + 10% O2 and 10% O2. On the non-impregnated cell stacks no NOx conversion was observed under any of the investigated conditions. However, BaO impregnation greatly enhanced the NOx conversion and at 400 °C and 9 V polarisation a BaO-impregnated cell stack showed 60% NOx conversion into N2 with 8% current efficiency in 1000 ppm NO + 10% O2. This demonstrates high NOx conversion can be achieved on an entirely ceramic cell without expensive noble metals. Furthermore the NOx conversion and current efficiency was shown to be strongly dependent on temperature and polarisation. The impedance analysis revealed that the BaO-impregnation increased the overall activity of the cell stacks, but also changed the adsorption state of NOx on the electrodes; whether the increased activity or the changed adsorption state is mainly responsible for the improved NOx conversion remains unknown.

 Please wait while we load your content...
Please wait while we load your content...